Targeting the Glucocorticoid Receptors During Alcohol Withdrawal to Reduce Protracted Neurocognitive Disorders
- PMID: 31620025
- PMCID: PMC6759466
- DOI: 10.3389/fpsyt.2019.00580
Targeting the Glucocorticoid Receptors During Alcohol Withdrawal to Reduce Protracted Neurocognitive Disorders
Abstract
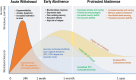
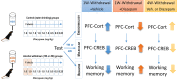
Persistent regional glucocorticoid (GC) dysregulation in alcohol-withdrawn subjects emerges as a key factor responsible for protracted molecular and neural alterations associated with long-term cognitive dysfunction. Regional brain concentrations of corticosterone vary independently from plasma concentrations in alcohol-withdrawn subjects, which may account for the treatment of alcohol withdrawal-induced persistent pathology. Thus, from a pharmacological point of view, a main issue remains to determine the relative efficacy of compounds targeting the GC receptors to attenuate or suppress the long-lasting persistence of brain regional GC dysfunctions in abstinent alcoholics, as well as persistent changes of neural plasticity. Data from animal research show that acting directly on GC receptors during the withdrawal period, via selective antagonists, can significantly counteract the development and persistence of cognitive and neural plasticity disorders during protracted abstinence. A critical remaining issue is to better assess the relative long-term efficacy of GC antagonists and other compounds targeting the corticotropic axis activity such as gamma-aminobutyric acid A (GABAA) and GABAB agonists. Indeed, benzodiazepines (acting indirectly on GABAA receptors) and baclofen (agonist of the GABAB receptor) are the compounds most widely used to reduce alcohol dependence. Clinical and preclinical data suggest that baclofen exerts an effective and more powerful counteracting action on such persistent cognitive and endocrine dysfunctions as compared to diazepam, even though its potential negative effects on memory processes, particularly at high doses, should be better taken into account.
Keywords: alcohol withdrawal and relapse; baclofen; benzodiazepines; corticosterone; gaba receptors; glucocorticoids; prefrontal cortex; working memory.
Copyright © 2019 Béracochéa, Mons and David.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

