Protocol for AREST: Apixaban for Early Prevention of Recurrent Embolic Stroke and Hemorrhagic Transformation-A Randomized Controlled Trial of Early Anticoagulation After Acute Ischemic Stroke in Atrial Fibrillation
- PMID: 31620067
- PMCID: PMC6763567
- DOI: 10.3389/fneur.2019.00975
Protocol for AREST: Apixaban for Early Prevention of Recurrent Embolic Stroke and Hemorrhagic Transformation-A Randomized Controlled Trial of Early Anticoagulation After Acute Ischemic Stroke in Atrial Fibrillation
Abstract
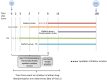
Background: Optimal timing to initiate anticoagulation after acute ischemic stroke (AIS) from atrial fibrillation (AF) is currently unknown. Compared to other stroke etiologies, AF typically provokes larger infarct volumes and greater concern of hemorrhagic transformation, so seminal randomized trials waited weeks to months to begin anticoagulation after initial stroke. Subsequent data are limited and non-randomized. Guidelines suggest anticoagulation initiation windows between 3 and 14 days post-stroke, with Class IIa recommendations, and level of evidence B in the USA and C in Europe. Aims: This open-label, parallel-group, multi-center, randomized controlled trial AREST (Apixaban for Early Prevention of Recurrent Embolic Stroke and Hemorrhagic Transformation) is designed to evaluate the safety and efficacy of early anticoagulation, based on stroke size, secondary prevention of ischemic stroke, and risks of subsequent hemorrhagic transformation. Methods: Subjects are randomly assigned in a 1:1 ratio to receive early apixaban at day 0-3 for transient ischemic attack (TIA), 3-5 for small-sized AIS (<1.5 cm), and 7-9 for medium-sized AIS (1.5 cm or greater but less than a full cortical territory), or warfarin at 1 week post-TIA or 2 weeks post-stroke. Large AISs are excluded. Study Outcomes: Primary: recurrent ischemic stroke, TIA, and fatal stroke; secondary: intracranial hemorrhage (ICH); hemorrhagic transformation (HT) of ischemic stroke; cerebral microbleeds (CMBs); neurologic disability [e.g., modified Rankin Scores (mRS), National Institutes of Health Stroke Scale (NIHSS), Stroke Specific Quality of Life scale (SS-QOL)]; and cardiac biomarkers [e.g., AF burden, transthoracic echo (TTE)/transesophageal echo (TEE) abnormalities]. Sample Size Estimates: Enrollment goal was 120 for 80% power (two-sided type I error rate of 0.05) to detect an absolute risk reduction of 16.5% postulated to occur with apixaban in the primary composite outcome of fatal stroke/recurrent ischemic stroke/TIA within 180 days. Enrollment was suspended at 91 subjects in 2019 after a focused guideline update recommended direct oral anticoagulants (DOACs) over warfarin in AF, excepting valvular disease (Class I, level of evidence A). Discussion: AREST will offer randomized controlled trial data about timeliness and safety of anticoagulation in AIS patients with AF. Clinical Trial Registration: www.ClinicalTrials.gov, identifier NCT02283294.
Keywords: acute ischemic stroke; anticoagulation timing; apixaban; atrial fibrillation; direct oral anticoagulant; transient ischemic attack.
Copyright © 2019 Rose, Meriwether, Fradley, Renati, Martin, Kasprowicz, Patel, Mokin, Murtagh, Kip, Bozeman, McTigue, Hilker, Kirby, Wick, Tran, Burgin and Labovitz.
Figures
References
-
- Lansberg MG, O'Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, et al. . Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic therapy and prevention of thrombosis, 9th ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (2012) 141:e601S−36S. 10.1378/chest.141.4.1129b - DOI - PMC - PubMed
-
- Kernan WN, American Heart Association Stroke Council Council on Cardiovascular and Stroke Nursing Council on Clinical Cardiology Council on Peripheral Vascular Disease et al. . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. 10.1161/STR.0000000000000024 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

