Complement in Human Pre-implantation Embryos: Attack and Defense
- PMID: 31620138
- PMCID: PMC6759579
- DOI: 10.3389/fimmu.2019.02234
Complement in Human Pre-implantation Embryos: Attack and Defense
Abstract
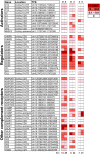
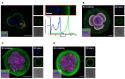
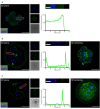
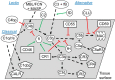
It is essential for early human life that mucosal immunological responses to developing embryos are tightly regulated. An imbalance of the complement system is a common feature of pregnancy complications. We hereby present the first full analysis of the expression and deposition of complement molecules in human pre-implantation embryos. Thus, far, immunological imbalance has been considered in stages of pregnancy following implantation. We here show that complement activation against developing human embryos takes place already at the pre-implantation stage. Using confocal microscopy, we observed deposition of activation products on healthy developing embryos, which highlights the need for strict complement regulation. We show that embryos express complement membrane inhibitors and bind soluble regulators. These findings show that mucosal complement targets human embryos, and indicate potential adverse pregnancy outcomes, if regulation of activation fails. In addition, single-cell RNA sequencing revealed cellular expression of complement activators. This shows that the embryonic cells themselves have the capacity to express and activate C3 and C5. The specific local embryonic expression of complement components, regulators, and deposition of activation products on the surface of embryos suggests that complement has immunoregulatory functions and furthermore may impact cellular homeostasis and differentiation at the earliest stages of life.
Keywords: complement; development; embryo; mucosal immunology; pre-implantation; reproductive immunology.
Copyright © 2019 Reichhardt, Lundin, Lokki, Recher, Vuoristo, Katayama, Tapanainen, Kere, Meri and Tuuri.
Figures

References
-
- Tedesco F, Narchi G, Radillo O, Meri S, Ferrone S, Betterle C. Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. J Immunol. (1993) 151:1562–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

