Preclinical Development of PQR514, a Highly Potent PI3K Inhibitor Bearing a Difluoromethyl-Pyrimidine Moiety
- PMID: 31620236
- PMCID: PMC6792169
- DOI: 10.1021/acsmedchemlett.9b00333
Preclinical Development of PQR514, a Highly Potent PI3K Inhibitor Bearing a Difluoromethyl-Pyrimidine Moiety
Abstract
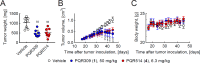
The phosphoinositide 3-kinase (PI3K)/mechanistic target of rapamycin (mTOR) pathway is a critical regulator of cell growth and is frequently hyperactivated in cancer. Therefore, PI3K inhibitors represent a valuable asset in cancer therapy. Herein we have developed a novel anticancer agent, the potent pan-PI3K inhibitor PQR514 (4), which is a follow-up compound for the phase-II clinical compound PQR309 (1). Compound 4 has an improved potency both in vitro and in cellular assays with respect to its predecessor compounds. It shows superiority in the suppression of cancer cell proliferation and demonstrates significant antitumor activity in an OVCAR-3 xenograft model at concentrations approximately eight times lower than PQR309 (1). The favorable pharmacokinetic profile and a minimal brain penetration promote PQR514 (4) as an optimized candidate for the treatment of systemic tumors.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): F.B., P.He., P.Hi., and D.F. are current or past employees of PIQUR Therapeutics AG, Basel, and P.He., D.F., and M.P.W. are shareholders of PIQUR Therapeutics AG.
Figures

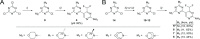


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

