Building Bridges in a Series of Estrogen Receptor Degraders: An Application of Metathesis in Medicinal Chemistry
- PMID: 31620239
- PMCID: PMC6792171
- DOI: 10.1021/acsmedchemlett.9b00370
Building Bridges in a Series of Estrogen Receptor Degraders: An Application of Metathesis in Medicinal Chemistry
Abstract
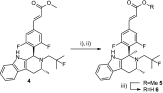
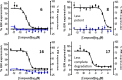
Herein we report the use of metathesis to construct a novel tetracyclic core in a series of estrogen receptor degraders. This improved the chemical stability, as assessed using an NMR-MS based assay, and gave a molecule with excellent physicochemical properties and pharmacokinetics in rat. X-ray crystallography established minimal perturbation of the bridged compounds relative to the unbridged analogues in the receptor binding pocket. Unfortunately, despite retaining excellent binding to ERα, this adversely affected the ability of the compounds to degrade the receptor.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- De Savi C.; Bradbury R. H.; Rabow A. A.; Norman R. A.; de Almeida C.; Andrews D. M.; Ballard P.; Buttar D.; Callis R. J.; Currie G. S.; Curwen J. O.; Davies C. D.; Donald C. S.; Feron L. J. L.; Gingell H.; Glossop S. C.; Hayter B. R.; Hussain S.; Karoutchi G.; Lamont S. G.; MacFaul P.; Moss T. A.; Pearson S. E.; Tonge M.; Walker G. E.; Weir H. M.; Wilson Z. Optimization of a novel binding motif to (E)-3-(3,5-difluoro-4-((1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid (AZD9496), a potent and orally bioavailable selective estrogen receptor downregulator and antagonist. J. Med. Chem. 2015, 58 (20), 8128–8140. 10.1021/acs.jmedchem.5b00984. - DOI - PubMed
-
- Tria G. S.; Abrams T.; Baird J.; Burks H. E.; Firestone B.; Gaither L. A.; Hamann L. G.; He G.; Kirby C. A.; Kim S.; Lombardo F.; Macchi K. J.; McDonnell D. P.; Mishina Y.; Norris J. D.; Nunez J.; Springer C.; Sun Y.; Thomsen N. M.; Wang C.; Wang J.; Yu B.; Tiong-Yip C.-L.; Peukert S. Discovery of LSZ102, a potent, orally bioavailable selective estrogen receptor degrader (SERD) for the treatment of estrogen receptor positive breast cancer. J. Med. Chem. 2018, 61 (7), 2837–2864. 10.1021/acs.jmedchem.7b01682. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

