A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies
- PMID: 31620481
- PMCID: PMC6789202
- DOI: 10.21037/sci.2019.08.11
A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies
Abstract
Multipotent mesenchymal stem cells (MSCs) have been considerably inspected as effective tool for cell-based therapy of inflammatory, immune-mediated, and degenerative diseases, attributed to their immunomodulatory, immunosuppressive, and regenerative potentials. In the present review, we focus on recent research findings of the clinical applications and therapeutic potential of this cell type, MSCs' mechanisms of therapy, strategies to improve their therapeutic potentials such as manipulations and preconditioning, and potential/unexpected risks which should be considered as a prerequisite step before clinical use. The potential risks would probably include undesirable immune responses, tumor formation and the transmission of incidental agents. Then, we also review some of the milestones in the field, briefly discuss challenges and highlight the new guideline suggested for future directions and perspectives.
Keywords: Mesenchymal stem cells (MSCs); genetic manipulation; potential risks; preconditioning.
2019 Stem Cell Investigation. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
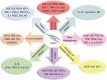
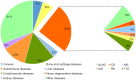
References
-
- Chen C. From mesenchymal stem cell therapy to discovery of drug therapy for systemic sclerosis. University of Southern California, ProQuest Dissertations Publishing, 2014. 3628136.
-
- Hirvonen T. Glycan Binding Proteins in Therapeutic Mesenchymal Stem Cell Research. 2014. Available online: https://helda.helsinki.fi/bitstream/handle/10138/135978/glycanbi.pdf?seq...
-
- Javaregowda PK, Yoon JW, Jang G. Roles of Mesenchymal Stem Cells (MSCs) in bacterial diseases. J Biomed Res 2013;14:184-94. 10.12729/jbr.2013.14.4.184 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
