A network of trans-cortical capillaries as mainstay for blood circulation in long bones
- PMID: 31620676
- PMCID: PMC6795552
- DOI: 10.1038/s42255-018-0016-5
A network of trans-cortical capillaries as mainstay for blood circulation in long bones
Abstract
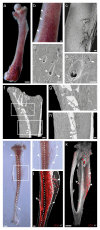
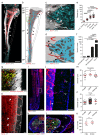
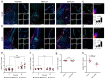
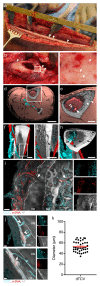
Closed circulatory systems (CCS) underlie the function of vertebrate organs, but in long bones their structure is unclear, although they constitute the exit route for bone marrow (BM) leukocytes. To understand neutrophil emigration from BM, we studied the vascular system of murine long bones. Here we show that hundreds of capillaries originate in BM, cross murine cortical bone perpendicularly along the shaft and connect to the periosteal circulation. Structures similar to these trans-cortical-vessels (TCVs) also exist in human limb bones. TCVs express arterial or venous markers and transport neutrophils. Furthermore, over 80% arterial and 59% venous blood passes through TCVs. Genetic and drug-mediated modulation of osteoclast count and activity leads to substantial changes in TCV numbers. In a murine model of chronic arthritic bone inflammation, new TCVs develop within weeks. Our data indicate that TCVs are a central component of the CCS in long bones and may represent an important route for immune cell export from the BM.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures


References
-
- Faury G. Function-structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol Biol (Paris) 2001;49:310–325. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

