High-throughput compound screen reveals mTOR inhibitors as potential therapeutics to reduce (auto)antibody production by human plasma cells
- PMID: 31621069
- PMCID: PMC6972998
- DOI: 10.1002/eji.201948241
High-throughput compound screen reveals mTOR inhibitors as potential therapeutics to reduce (auto)antibody production by human plasma cells
Abstract
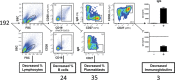
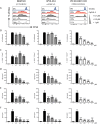
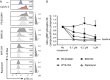
Antibody production by the B cell compartment is a crucial part of the adaptive immune response. Dysregulated antibody production in the form of autoantibodies can cause autoimmune disease. To date, B-cell depletion with anti-CD20 antibodies is commonly applied in autoimmunity, but pre-existing plasma cells are not eliminated in this way. Alternative ways of more selective inhibition of antibody production would add to the treatment of these autoimmune diseases. To explore novel therapeutic targets in signaling pathways essential for plasmablast formation and/or immunoglobulin production, we performed a compound screen of almost 200 protein kinase inhibitors in a robust B-cell differentiation culture system. This study yielded 35 small cell-permeable compounds with a reproducible inhibitory effect on B-cell activation and plasmablast formation, among which was the clinically applied mammalian target of rapamycin (mTOR) inhibitor rapamycin. Two additional compounds targeting the phosphoinositide 3-kinase-AKT-mTOR pathway (BKM120 and WYE-354) did not affect proliferation and plasmablast formation, but specifically reduced the immunoglobulin production. With this compound screen we successfully applied a method to investigate therapeutic targets for B-cell differentiation and identified compounds in the phosphoinositide 3-kinase-AKT-mTOR pathway that could specifically inhibit immunoglobulin production only. These drugs may well be explored to be of value in current B-cell-depleting treatment regimens in autoimmune disorders.
Keywords: B cells; B-cell activation and differentiation; PI3K-AKT-mTOR; autoimmune disease; plasma cells.
© 2019 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures


References
-
- Durandy, A. , Kracker, S. and Fischer, A. , Primary antibody deficiencies. Nat. Rev. Immunol. 2013. 13: 519–533. - PubMed
-
- aan de Kerk, D. J. , Jansen, M. H. , ten Berge, I. J. , van Leeuwen, E. M. and Kuijpers, T. W. , Identification of B cell defects using age‐defined reference ranges for in vivo and in vitro B cell differentiation. J. Immunol. 2013. 190: 5012–5019. - PubMed
-
- Nijwening, J. H. and Beijersbergen, R. L. , Using large‐scale RNAi screens to identify novel drug targets for cancer. IDrugs 2010. 13: 772–777. - PubMed
-
- Bernards, R. , Brummelkamp, T. R. and Beijersbergen, R. L. , shRNA libraries and their use in cancer genetics. Nat. Methods 2006. 3: 701–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

