Structurally Modified Cyclopenta[ b]benzofuran Analogues Isolated from Aglaia perviridis
- PMID: 31621322
- PMCID: PMC6819999
- DOI: 10.1021/acs.jnatprod.9b00631
Structurally Modified Cyclopenta[ b]benzofuran Analogues Isolated from Aglaia perviridis
Abstract
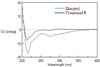
Four new cyclopenta[b]benzofuran derivatives based on an unprecedented carbon skeleton (1-4), with a dihydrofuran ring fused to dioxanyl and aryl rings, along with a new structural analogue (5) of 5‴-episilvestrol (episilvestrol, 7), were isolated from an aqueous extract of a large-scale re-collection of the roots of Aglaia perviridis collected in Vietnam. Compound 5 demonstrated mutarotation in solution due to the presence of a hydroxy group at C-2‴, leading to the isolation of a racemic mixture, despite being purified on a chiral-phase HPLC column. Silvestrol (6) and episilvestrol (7) were isolated from the most potently cytotoxic chloroform subfraction of the roots. All new structures were elucidated using 1D and 2D NMR, HRESIMS, IR, UV, and ECD spectroscopic data. Of the five newly isolated compounds, only compound 5 exhibited cytotoxic activity against a human colon cancer (HT-29) and human prostate cancer cell line (PC-3), with IC50 values of 2.3 μM in both cases. The isolated compounds (1-5) double the number of dioxanyl ring-containing rocaglate analogues reported to date from Aglaia species and present additional information on the structural requirements for cancer cell line cytotoxicity within this compound class.
Figures



Similar articles
-
Additional Bioactive Silvestrol Analogs from the Roots of Aglaia perviridis Collected in Vietnam.J Nat Prod. 2025 Mar 28;88(3):662-670. doi: 10.1021/acs.jnatprod.4c01247. Epub 2025 Mar 4. J Nat Prod. 2025. PMID: 40040236
-
Bioactive flavaglines and other constituents isolated from Aglaia perviridis.J Nat Prod. 2013 Mar 22;76(3):394-404. doi: 10.1021/np3007588. Epub 2013 Jan 9. J Nat Prod. 2013. PMID: 23301897 Free PMC article.
-
Cyclopenta[b]benzofuran and Secodammarane Derivatives from the Stems of Aglaia stellatopilosa.J Nat Prod. 2016 Apr 22;79(4):784-91. doi: 10.1021/acs.jnatprod.5b00810. Epub 2016 Mar 14. J Nat Prod. 2016. PMID: 26974604 Free PMC article.
-
Rocaglamide, silvestrol and structurally related bioactive compounds from Aglaia species.Nat Prod Rep. 2014 Jul;31(7):924-39. doi: 10.1039/c4np00006d. Epub 2014 May 2. Nat Prod Rep. 2014. PMID: 24788392 Free PMC article. Review.
-
Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy.Anticancer Agents Med Chem. 2006 Jul;6(4):319-45. doi: 10.2174/187152006777698123. Anticancer Agents Med Chem. 2006. PMID: 16842234 Review.
Cited by
-
Strategies for the discovery of potential anticancer agents from plants collected from Southeast Asian tropical rainforests as a case study.Nat Prod Rep. 2023 Jul 19;40(7):1181-1197. doi: 10.1039/d2np00080f. Nat Prod Rep. 2023. PMID: 37194649 Free PMC article. Review.
-
Stewartiacids A-N, C-23 carboxylated triterpenoids from Chinese Stewartia and their inhibitory effects against ATP-citrate lyase and NF-κB.RSC Adv. 2020 Jan 21;10(6):3343-3356. doi: 10.1039/c9ra09542j. eCollection 2020 Jan 16. RSC Adv. 2020. PMID: 35497717 Free PMC article.
-
Discovery of Anticancer Agents of Diverse Natural Origin.J Nat Prod. 2022 Mar 25;85(3):702-719. doi: 10.1021/acs.jnatprod.2c00036. Epub 2022 Feb 25. J Nat Prod. 2022. PMID: 35213158 Free PMC article.
-
Additional Bioactive Silvestrol Analogs from the Roots of Aglaia perviridis Collected in Vietnam.J Nat Prod. 2025 Mar 28;88(3):662-670. doi: 10.1021/acs.jnatprod.4c01247. Epub 2025 Mar 4. J Nat Prod. 2025. PMID: 40040236
-
Update on Phytochemical and Biological Studies on Rocaglate Derivatives from Aglaia Species.Planta Med. 2021 Oct;87(12-13):937-948. doi: 10.1055/a-1401-9562. Epub 2021 Mar 30. Planta Med. 2021. PMID: 33784769 Free PMC article. Review.
References
-
- King ML; Chiang C-C; Ling H-C; Fujita E; Ochiai M; McPhail AT J. Chem. Soc., Chem. Commun 1982, 1150–1151.
-
- Dumontet V; Thoison O; Omobuwajo OR; Martin M-T; Perromat G; Chiaroni A; Riche C; Pais M; Sevenet T. Tetrahedron 1996, 52, 6931–6942.
-
- Hwang BY; Su BN; Chai H-B; Mi Q; Kardono LBS; Afriastini JJ; Riswan S; Santarsiero BD; Mesecar AD; Wild R; Fairchild CR; Vite GD; Rose WC; Farnsworth NR; Cordell GA; Pezzuto JM; Swanson SM; Kinghorn AD J. Org. Chem 2004, 69, 3350–3358; ibid., 69, 6156. - PubMed
-
- Meurer-Grimes, B. M.; Yu, J.; Vairo, G. L. U.S. patent 6710075 B2, 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

