Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer
- PMID: 31621801
- PMCID: PMC6802034
- DOI: 10.1001/jamaoncol.2019.3616
Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer
Erratum in
-
Error in Figure 2D.JAMA Oncol. 2019 Dec 1;5(12):1811. doi: 10.1001/jamaoncol.2019.5667. JAMA Oncol. 2019. PMID: 31830217 Free PMC article. No abstract available.
Abstract
Importance: Adjuvant chemotherapy in patients with stage III colon cancer prevents recurrence by eradicating minimal residual disease. However, which patients remain at high risk of recurrence after completing standard adjuvant treatment cannot currently be determined. Postsurgical circulating tumor DNA (ctDNA) analysis can detect minimal residual disease and is associated with recurrence in colorectal cancers.
Objective: To determine whether serial postsurgical and postchemotherapy ctDNA analysis could provide a real-time indication of adjuvant therapy efficacy in stage III colon cancer.
Design, setting, and participants: This multicenter, Australian, population-based cohort biomarker study recruited 100 consecutive patients with newly diagnosed stage III colon cancer planned for 24 weeks of adjuvant chemotherapy from November 1, 2014, through May 31, 2017. Patients with another malignant neoplasm diagnosed within the last 3 years were excluded. Median duration of follow-up was 28.9 months (range, 11.6-46.4 months). Physicians were blinded to ctDNA results. Data were analyzed from December 10, 2018, through June 23, 2019.
Exposures: Serial plasma samples were collected after surgery and after chemotherapy. Somatic mutations in individual patients' tumors were identified via massively parallel sequencing of 15 genes commonly mutated in colorectal cancer. Personalized assays were designed to quantify ctDNA.
Main outcomes and measures: Detection of ctDNA and recurrence-free interval (RFI).
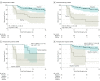
Results: After 4 exclusions, 96 eligible patients were eligible; median patient age was 64 years (range, 26-82 years); 49 (51%) were men. At least 1 somatic mutation was identified in the tumor tissue of all 96 evaluable patients. Circulating tumor DNA was detectable in 20 of 96 (21%) postsurgical samples and was associated with inferior recurrence-free survival (hazard ratio [HR], 3.8; 95% CI, 2.4-21.0; P < .001). Circulating tumor DNA was detectable in 15 of 88 (17%) postchemotherapy samples. The estimated 3-year RFI was 30% when ctDNA was detectable after chemotherapy and 77% when ctDNA was undetectable (HR, 6.8; 95% CI, 11.0-157.0; P < .001). Postsurgical ctDNA status remained independently associated with RFI after adjusting for known clinicopathologic risk factors (HR, 7.5; 95% CI, 3.5-16.1; P < .001).
Conclusions and relevance: Results suggest that ctDNA analysis after surgery is a promising prognostic marker in stage III colon cancer. Postchemotherapy ctDNA analysis may define a patient subset that remains at high risk of recurrence despite completing standard adjuvant treatment. This high-risk population presents a unique opportunity to explore additional therapeutic approaches.
Conflict of interest statement
Figures
Comment in
-
Is the Patient Cured?JAMA Oncol. 2019 Dec 1;5(12):1695-1697. doi: 10.1001/jamaoncol.2019.3612. JAMA Oncol. 2019. PMID: 31621795 No abstract available.
-
Circulating Tumor DNA as a Prognostic Marker in Stage III Colon Cancer.JAMA Oncol. 2020 Jun 1;6(6):932. doi: 10.1001/jamaoncol.2020.0283. JAMA Oncol. 2020. PMID: 32239189 No abstract available.
References
-
- André T, Boni C, Mounedji-Boudiaf L, et al. ; Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators . Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343-2351. doi:10.1056/NEJMoa032709 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

