Deleterious mtDNA mutations are common in mature oocytes
- PMID: 31621839
- PMCID: PMC7068114
- DOI: 10.1093/biolre/ioz202
Deleterious mtDNA mutations are common in mature oocytes
Abstract
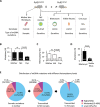
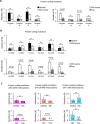
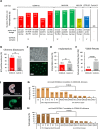
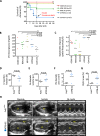
Heritable mitochondrial DNA (mtDNA) mutations are common, yet only a few recurring pathogenic mtDNA variants account for the majority of known familial cases in humans. Purifying selection in the female germline is thought to be responsible for the elimination of most harmful mtDNA mutations during oogenesis. Here we show that deleterious mtDNA mutations are abundant in ovulated mature mouse oocytes and preimplantation embryos recovered from PolG mutator females but not in their live offspring. This implies that purifying selection acts not in the maternal germline per se, but during post-implantation development. We further show that oocyte mtDNA mutations can be captured and stably maintained in embryonic stem cells and then reintroduced into chimeras, thereby allowing examination of the effects of specific mutations on fetal and postnatal development.
Keywords: mitochondria; mtDNA; oocyte.
© The Author(s) 2019. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
Maternal ageing impairs mitochondrial DNA kinetics during early embryogenesis in mice.Hum Reprod. 2019 Jul 8;34(7):1313-1324. doi: 10.1093/humrep/dez054. Hum Reprod. 2019. PMID: 31174209
-
Quantitative and qualitative changes of mitochondria in human preimplantation embryos.J Assist Reprod Genet. 2017 May;34(5):573-580. doi: 10.1007/s10815-017-0886-6. Epub 2017 Feb 11. J Assist Reprod Genet. 2017. PMID: 28190213 Free PMC article.
-
Differences in Strength and Timing of the mtDNA Bottleneck between Zebrafish Germline and Non-germline Cells.Cell Rep. 2016 Jul 19;16(3):622-30. doi: 10.1016/j.celrep.2016.06.023. Epub 2016 Jun 30. Cell Rep. 2016. PMID: 27373161
-
mtDNA mutations variously impact mtDNA maintenance throughout the human embryofetal development.Clin Genet. 2015 Nov;88(5):416-24. doi: 10.1111/cge.12557. Epub 2015 Feb 3. Clin Genet. 2015. PMID: 25523230 Review.
-
Mitochondrial DNA Assessment to Determine Oocyte and Embryo Viability.Semin Reprod Med. 2015 Nov;33(6):401-9. doi: 10.1055/s-0035-1567821. Epub 2015 Nov 13. Semin Reprod Med. 2015. PMID: 26565384 Review.
Cited by
-
Genetics of Oocyte Maturation Defects and Early Embryo Development Arrest.Genes (Basel). 2022 Oct 22;13(11):1920. doi: 10.3390/genes13111920. Genes (Basel). 2022. PMID: 36360157 Free PMC article. Review.
-
Pathogenic mitochondrial DNA 3243A>G mutation: From genetics to phenotype.Front Genet. 2022 Oct 6;13:951185. doi: 10.3389/fgene.2022.951185. eCollection 2022. Front Genet. 2022. PMID: 36276941 Free PMC article. Review.
-
Wenshen Yangxue decoction promotes follicular development in aged female mice stimulation of the silent information regulator 3/forkhead transcription factor O1 3a pathway.J Tradit Chin Med. 2022 Aug;42(4):539-545. doi: 10.19852/j.cnki.jtcm.20220617.004. J Tradit Chin Med. 2022. PMID: 35848970 Free PMC article.
-
Heteroplasmy and Individual Mitogene Pools: Characteristics and Potential Roles in Ecological Studies.Biology (Basel). 2023 Nov 20;12(11):1452. doi: 10.3390/biology12111452. Biology (Basel). 2023. PMID: 37998051 Free PMC article. Review.
-
Horizontal mtDNA transfer between cells is common during mouse development.iScience. 2022 Feb 10;25(3):103901. doi: 10.1016/j.isci.2022.103901. eCollection 2022 Mar 18. iScience. 2022. PMID: 35243258 Free PMC article.
References
-
- Wallace DC, Ye JH, Neckelmann SN, Singh G, Webster KA, Greenberg BD. Sequence analysis of cDNAs for the human and bovine ATP synthase beta subunit: Mitochondrial DNA genes sustain seventeen times more mutations. Curr Genet 1987; 12:81–90. - PubMed
-
- Kazak L, Reyes A, Holt IJ. Minimizing the damage: Repair pathways keep mitochondrial DNA intact. Nat Rev Mol Cell Biol 2012; 13:659–671. - PubMed
-
- Kang E, Wang X, Tippner-Hedges R, Ma H, Folmes Clifford DL, Gutierrez Nuria M, Lee Y, Van Dyken C, Ahmed R, Li Y, Koski A, Hayama T et al. . Age-related accumulation of somatic mitochondrial DNA mutations in adult-derived human iPSCs. Cell Stem Cell 2016; 18:625–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

