Enriching cancer pharmacology with drugs of marine origin
- PMID: 31621891
- PMCID: PMC6976878
- DOI: 10.1111/bph.14876
Enriching cancer pharmacology with drugs of marine origin
Abstract
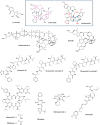
Marine natural products have proven, over the last half-century, to be effective biological modulators. These molecules have revealed new targets for cancer therapy as well as dissimilar modes of action within typical classes of drugs. In this scenario, innovation from marine-based pharmaceuticals has helped advance cancer chemotherapy in many aspects, as most of these are designated as first-in-class drugs. Here, by examining the path from discovery to development of clinically approved drugs of marine origin for cancer treatment-cytarabine (Cytosar-U®), trabectedin (Yondelis®), eribulin (Halaven®), brentuximab vedotin (Adcetris®), and plitidepsin (Aplidin®)- together with those in late clinical trial phases-lurbinectedin, plinabulin, marizomib, and plocabulin-the present review offers a critical analysis of the contributions given by these new compounds to cancer pharmacotherapy.
© 2019 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ahuja, D. , Vera, M. D. , SirDeshpande, B. V. , Morimoto, H. , Williams, P. G. , Joullié, M. M. , et al. (2000). Inhibition of protein synthesis by didemnin B: How EF‐1α mediates inhibition of translocation. Biochemistry, 39, 4339–4346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FCT/MCTES (UID/Mult/04378/2019)/Applied Molecular Biosciences Unit - UCIBIO/International
- Conselho Nacional de Desenvolvimento Científico e Tecnológico/International
- IF/00700/2014 to SPG/Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa/International
- 2015/17177-6/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- 2017/09022-8/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- 2017/17648-4/Fundação de Amparo à Pesquisa do Estado de São Paulo/International
- ArboControl Brasil Project FNS/UnB TED42/2017/Ministério da Saúde/International
- ArboControl Brasil Project FNS/UnB TED74/2016/Ministério da Saúde/International
- UID/Multi/04378/2019/FCT/MCTES/International
- 465637/2014-0/National Institute of Science and Technology - INCTBioNat/International
- TED42/2017/ArboControl Brasil Project/International
- FNS/UnB TED74/2016/ArboControl Brasil Project/International
- CNPQ/International
- 2017/17648-4/FAPESP/International
- 2017/09022-8/FAPESP/International
- 2015/17177-6/FAPESP/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

