GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevent primary tumor growth and metastasis in Ewing sarcoma
- PMID: 31621900
- PMCID: PMC7440656
- DOI: 10.1002/ijc.32743
GD2-directed CAR-T cells in combination with HGF-targeted neutralizing antibody (AMG102) prevent primary tumor growth and metastasis in Ewing sarcoma
Abstract
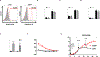
Ewing sarcoma (EWS) is the second most common and aggressive type of metastatic bone tumor in adolescents and young adults. There is unmet medical need to develop and test novel pharmacological targets and novel therapies to treat EWS. Here, we found that EWS expresses high levels of a p53 isoform, delta133p53. We further determined that aberrant expression of delta133p53 induced HGF secretion resulting in tumor growth and metastasis. Thereafter, we evaluated targeting EWS tumors with HGF receptor neutralizing antibody (AMG102) in preclinical studies. Surprisingly, we found that targeting EWS tumors with HGF receptor neutralizing antibody (AMG102) in combination with GD2-specific, CAR-reengineered T-cell therapy synergistically inhibited primary tumor growth and establishment of metastatic disease in preclinical models. Furthermore, our data suggested that AMG102 treatment alone might increase leukocyte infiltration including efficient CAR-T access into tumor mass and thereby improves its antitumor activity. Together, our findings warrant the development of novel CAR-T-cell therapies that incorporate HGF receptor neutralizing antibody to improve therapeutic potency, not only in EWS but also in tumors with aberrant activation of the HGF/c-MET pathway.
Keywords: AMG102; CAR-T cell therapy; Ewing sarcoma; HGF; delta133p53; metastasis; preclinical studies.
© 2019 UICC.
Conflict of interest statement
Figures
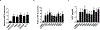
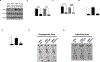

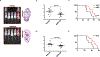

Similar articles
-
Tumor-derived G-CSF induces an immunosuppressive microenvironment in an osteosarcoma model, reducing response to CAR.GD2 T-cells.J Hematol Oncol. 2024 Dec 18;17(1):127. doi: 10.1186/s13045-024-01641-7. J Hematol Oncol. 2024. PMID: 39695851 Free PMC article.
-
VEGFR2 as a target for CAR T cell therapy of Ewing sarcoma.Pediatr Blood Cancer. 2020 Oct;67(10):e28313. doi: 10.1002/pbc.28313. Epub 2020 Jul 30. Pediatr Blood Cancer. 2020. PMID: 32729251
-
EZH2 Inhibition in Ewing Sarcoma Upregulates GD2 Expression for Targeting with Gene-Modified T Cells.Mol Ther. 2019 May 8;27(5):933-946. doi: 10.1016/j.ymthe.2019.02.014. Epub 2019 Feb 23. Mol Ther. 2019. PMID: 30879952 Free PMC article.
-
Therapeutic opportunities in Ewing sarcoma: EWS-FLI inhibition via LSD1 targeting.Oncotarget. 2016 Apr 5;7(14):17616-30. doi: 10.18632/oncotarget.7124. Oncotarget. 2016. PMID: 26848860 Free PMC article. Review.
-
A Novel Treatment for Ewing's Sarcoma: Chimeric Antigen Receptor-T Cell Therapy.Front Immunol. 2021 Sep 10;12:707211. doi: 10.3389/fimmu.2021.707211. eCollection 2021. Front Immunol. 2021. PMID: 34566963 Free PMC article. Review.
Cited by
-
The hepatocyte growth factor/mesenchymal epithelial transition factor axis in high-risk pediatric solid tumors and the anti-tumor activity of targeted therapeutic agents.Front Pediatr. 2022 Aug 10;10:910268. doi: 10.3389/fped.2022.910268. eCollection 2022. Front Pediatr. 2022. PMID: 36034555 Free PMC article. Review.
-
The efficacy and applicability of chimeric antigen receptor (CAR) T cell-based regimens for primary bone tumors: A comprehensive review of current evidence.J Bone Oncol. 2024 Sep 22;48:100635. doi: 10.1016/j.jbo.2024.100635. eCollection 2024 Oct. J Bone Oncol. 2024. PMID: 39381633 Free PMC article. Review.
-
Dissecting Immunotherapy Strategies for Small Cell Lung Cancer: Antibodies, Ionizing Radiation and CAR-T.Int J Mol Sci. 2022 Oct 22;23(21):12728. doi: 10.3390/ijms232112728. Int J Mol Sci. 2022. PMID: 36361523 Free PMC article. Review.
-
Sphingolipids and Lymphomas: A Double-Edged Sword.Cancers (Basel). 2022 Apr 19;14(9):2051. doi: 10.3390/cancers14092051. Cancers (Basel). 2022. PMID: 35565181 Free PMC article. Review.
-
Management of Unresectable Localized Pelvic Bone Sarcomas: Current Practice and Future Perspectives.Cancers (Basel). 2022 May 22;14(10):2546. doi: 10.3390/cancers14102546. Cancers (Basel). 2022. PMID: 35626150 Free PMC article. Review.
References
-
- Esiashvili N, Goodman M, Marcus RB, Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 2008;30: 425–430. - PubMed
-
- Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer 2002;2: 289–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

