Transplantation of adipose-derived mesenchymal stem cell sheets directly into the kidney suppresses the progression of renal injury in a diabetic nephropathy rat model
- PMID: 31622047
- PMCID: PMC7232293
- DOI: 10.1111/jdi.13164
Transplantation of adipose-derived mesenchymal stem cell sheets directly into the kidney suppresses the progression of renal injury in a diabetic nephropathy rat model
Abstract
Aims/introduction: Adipose-derived mesenchymal stem cell (ASC) transplantation is a promising therapy for diabetic nephropathy (DN). However, intravascular administration of ASCs is associated with low engraftment in target organs. Therefore, we considered applying the cell sheet technology to ASCs. In this study, ASC sheets were directly transplanted into the kidneys of a DN rat model, and therapeutic consequences were analyzed.
Materials and methods: Adipose-derived mesenchymal stem cells were isolated from adipose tissues of 7-week-old enhanced green fluorescent protein rats, and ASC sheets were prepared using a temperature-responsive culture dish. A DN rat model was established from 5-week-old Spontaneously Diabetic Torii fatty rats. Seven-week-old DN rats (n = 21) were assigned to one of the following groups: sham-operated (n = 6); ASC suspension (6.0 × 106 cells/mL) administered intravenously (n = 7); six ASC sheets transplanted directly into the kidney (n = 8). The therapeutic effect of the cell sheets was determined based on urinary biomarker expression and histological analyses.
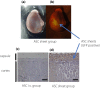
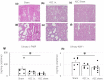
Results: The ASC sheets survived under the kidney capsule of the DN rat model for 14 days after transplantation. Furthermore, albuminuria and urinary tumor necrosis factor-α levels were significantly lower in the ASC sheets transplanted directly into the kidney group than in the sham-operated and ASC suspension administered intravenously groups (P < 0.05). Histologically, the ASC sheets transplanted directly into the kidney group presented mild atrophy of the proximal tubule and maintained the renal tubular structure.
Conclusions: Transplantation of ASC sheets directly into the kidney improved transplantation efficiency and suppressed renal injury progression. Therefore, the ASC sheet technology might be a promising novel treatment for DN.
Keywords: Adipose-derived mesenchymal stem cell; Cell sheet; Renal injury.
© 2019 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Tatsuya Shimizu was a member of the scientific advisory board and a shareholder of CellSeed, Inc. Tokyo Women’s Medical University received research funding from CellSeed, Inc. The other authors declare no conflict of interest.
Figures




References
-
- Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 2008; 4: 444–452. - PubMed
-
- Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep 2006; 6: 463–468. - PubMed
-
- Navarro‐González JF, Mora‐Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008; 19: 433–442. - PubMed
-
- Dressler RL. Antihypertensive agents for prevention of diabetic nephropathy. Am Fam Physician 2006; 74: 77–79. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

