Viral quasispecies
- PMID: 31622336
- PMCID: PMC6797082
- DOI: 10.1371/journal.pgen.1008271
Viral quasispecies
Abstract
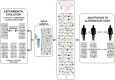
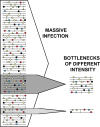
Viral quasispecies refers to a population structure that consists of extremely large numbers of variant genomes, termed mutant spectra, mutant swarms or mutant clouds. Fueled by high mutation rates, mutants arise continually, and they change in relative frequency as viral replication proceeds. The term quasispecies was adopted from a theory of the origin of life in which primitive replicons) consisted of mutant distributions, as found experimentally with present day RNA viruses. The theory provided a new definition of wild type, and a conceptual framework for the interpretation of the adaptive potential of RNA viruses that contrasted with classical studies based on consensus sequences. Standard clonal analyses and deep sequencing methodologies have confirmed the presence of myriads of mutant genomes in viral populations, and their participation in adaptive processes. The quasispecies concept applies to any biological entity, but its impact is more evident when the genome size is limited and the mutation rate is high. This is the case of the RNA viruses, ubiquitous in our biosphere, and that comprise many important pathogens. In virology, quasispecies are defined as complex distributions of closely related variant genomes subjected to genetic variation, competition and selection, and that may act as a unit of selection. Despite being an integral part of their replication, high mutation rates have an upper limit compatible with inheritable information. Crossing such a limit leads to RNA virus extinction, a transition that is the basis of an antiviral design termed lethal mutagenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Eigen M, Schuster P (1979) The hypercycle. A principle of natural self-organization Berlin: Springer. - PubMed

