High Rates of Drug-induced Liver Injury in People Living With HIV Coinfected With Tuberculosis (TB) Irrespective of Antiretroviral Therapy Timing During Antituberculosis Treatment: Results From the Starting Antiretroviral Therapy at Three Points in TB Trial
- PMID: 31622456
- PMCID: PMC7931836
- DOI: 10.1093/cid/ciz732
High Rates of Drug-induced Liver Injury in People Living With HIV Coinfected With Tuberculosis (TB) Irrespective of Antiretroviral Therapy Timing During Antituberculosis Treatment: Results From the Starting Antiretroviral Therapy at Three Points in TB Trial
Abstract
Background: New onset or worsening drug-induced liver injury challenges coinfected patients on antiretroviral therapy (ART) initiation during antituberculosis (TB) treatment.
Methods: Post hoc analysis within a randomized trial, the Starting Antiretroviral Therapy at Three Points in Tuberculosis trial, was conducted. Patients were randomized to initiate ART either early or late during TB treatment or after TB treatment completion. Liver enzymes were measured at baseline, 6-month intervals, and when clinically indicated.
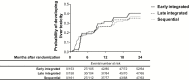
Results: Among 642 patients enrolled, the median age was 34 years (standard deviation, 28-40), and 17.6% had baseline CD4+ cell counts <50 cells/mm3. Overall, 146/472 patients (52, 47, and 47: early, late, and sequential arms) developed new-onset liver injury following TB treatment initiation. The incidence of liver injury post-ART initiation in patients with CD4+ cell counts <200 cells/mm3 and ≥200 cells/ mm3 was 27.4 (95% confidence interval [CI], 18.0-39.8), 19.0 (95% CI, 10.9-30.9), and 18.4 (95% CI, 8.8-33.8) per 100 person-years, and 32.1 (95% CI, 20.1-48.5), 11.8 (95% CI, 4.3-25.7), and 28.2 (95% CI, 13.5-51.9) per 100 person-years in the early, late integrated, and sequential treatment arms, respectively. Severe and life-threatening liver injury occurred in 2, 7, and 3 early, late, and sequential treatment arm patients, respectively. Older age and hepatitis B positivity predicted liver injury.
Conclusions: High incidence rates of liver injury among cotreated human immunodeficiency virus (HIV)-TB coinfected patients were observed. Clinical guidelines and policies must provide guidance on frequency of liver function monitoring for HIV-TB coinfected patients.
Keywords: HIV–TB integration; South Africa; antiretroviral treatment; liver injury; tuberculosis treatment.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- World Health Organization. Global Tuberculosis report. 2018. Geneva, Switzerland: WHO.
-
- Jong E, Conradie F, Black A, Menezes C, John M, Meintjes G. Consensus statement: management of drug-induced liver injury in HIV-positive patients treated for TB: guideline. South Afr J HIV Med 2013; 14:113–9.
-
- Hoffmann CJ, Charalambous S, Thio CL, et al. . Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS 2007; 21:1301–8. - PubMed
-
- Perriëns JH, St Louis ME, Mukadi YB, et al. . Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med 1995; 332:779–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials