Reproductive tract biology: Of mice and men
- PMID: 31622789
- PMCID: PMC7339118
- DOI: 10.1016/j.diff.2019.07.004
Reproductive tract biology: Of mice and men
Abstract
The study of male and female reproductive tract development requires expertise in two separate disciplines, developmental biology and endocrinology. For ease of experimentation and economy, the mouse has been used extensively as a model for human development and pathogenesis, and for the most part similarities in developmental processes and hormone action provide ample justification for the relevance of mouse models for human reproductive tract development. Indeed, there are many examples describing the phenotype of human genetic disorders that have a reasonably comparable phenotype in mice, attesting to the congruence between mouse and human development. However, anatomic, developmental and endocrinologic differences exist between mice and humans that (1) must be appreciated and (2) considered with caution when extrapolating information between all animal models and humans. It is critical that the investigator be aware of both the similarities and differences in organogenesis and hormone action within male and female reproductive tracts so as to focus on those features of mouse models with clear relevance to human development/pathology. This review, written by a team with extensive expertise in the anatomy, developmental biology and endocrinology of both mouse and human urogenital tracts, focusses upon the significant human/mouse differences, and when appropriate voices a cautionary note regarding extrapolation of mouse models for understanding development of human male and female reproductive tracts.
Keywords: Adenosis; Alpha-fetoprotein; Benign prostatic hyperplasia; Clitoris; Hypospadias; Mullerian duct; Penis; Prepuce; Prostate; Uterus; Vagina.
Copyright © 2019 International Society of Differentiation. Published by Elsevier B.V. All rights reserved.
Figures

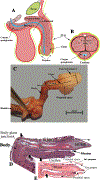
 . (D) Mid- sagittal hematoxylin–eosin stained section of the adult mouse penis with the external prepuce removed. (E) The mouse glans penis within the proximal portion of the external prepuce. Note reflection of epithelium of the external prepuce onto the surface of the penis indicated by the large solid arrows in both (D & E). (Adapted from Rodriguez et al. 2011 and Cunha et al. 2015 with permission).
. (D) Mid- sagittal hematoxylin–eosin stained section of the adult mouse penis with the external prepuce removed. (E) The mouse glans penis within the proximal portion of the external prepuce. Note reflection of epithelium of the external prepuce onto the surface of the penis indicated by the large solid arrows in both (D & E). (Adapted from Rodriguez et al. 2011 and Cunha et al. 2015 with permission).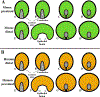


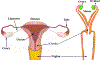
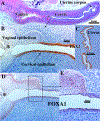

References
-
- Aboseif S, El-Sakka A, Young P and Cunha G (1999) Mesenchymal reprogramming of adult human epithelial differentiation. Differentiation 65:113–118. - PubMed
-
- Atkins KA (2019) Normal Histology of the Uterus and Fallopian Tubes In: Mills S (ed) Histology for Pathologists. Lippincott, Philadelphia, pp 1059–1106.
-
- Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J and Rowley DR (2003) Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res 9:4792–4801. - PubMed

