Chain Breaking Antioxidant Activity of Heavy (S, Se, Te) Chalcogens Substituted Polyphenols
- PMID: 31623080
- PMCID: PMC6826409
- DOI: 10.3390/antiox8100487
Chain Breaking Antioxidant Activity of Heavy (S, Se, Te) Chalcogens Substituted Polyphenols
Abstract
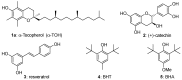
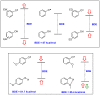
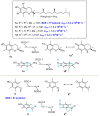
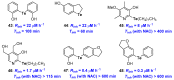
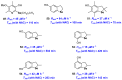
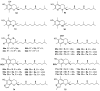
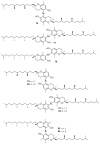
Polyphenols are probably the most important family of natural and synthetic chain-breaking antioxidants. Since long ago, chemists have studied how structural (bioinspired) modifications can improve the antioxidant activity of these compounds in terms of reaction rate with radical reactive oxygen species (ROS), catalytic character, multi-defence action, hydrophilicity/lipophilicity, biodistribution etc. In this framework, we will discuss the effect played on the overall antioxidant profile by the insertion of heavy chalcogens (S, Se and Te) in the phenolic skeleton.
Keywords: chain breaking antioxidants; polyphenols; selenium; sulfur; tellurium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
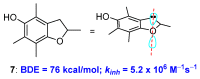
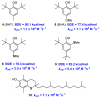


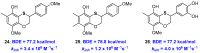


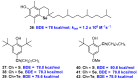

References
-
- Ingold K.U. Inhibition of the Autoxidation of Organic Substances in the Liquid Phase. Chem. Rev. 1961;61:563–589. doi: 10.1021/cr60214a002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

