The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs
- PMID: 31623120
- PMCID: PMC6835408
- DOI: 10.3390/polym11101692
The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs
Abstract
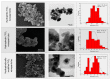
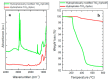
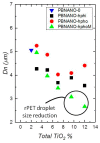
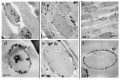
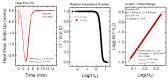
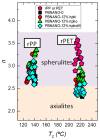
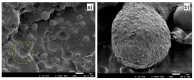
Titanium dioxide (TiO2) nanoparticles have recently appeared in PET waste because of the introduction of opaque PET bottles. We prepare polymer blend nanocomposites (PBNANOs) by adding hydrophilic (hphi), hydrophobic (hpho), and hydrophobically modified (hphoM) titanium dioxide (TiO2) nanoparticles to 80rPP/20rPET recycled blends. Contact angle measurements show that the degree of hydrophilicity of TiO2 decreases in the order hphi > hpho > hphoM. A reduction of rPET droplet size occurs with the addition of TiO2 nanoparticles. The hydrophilic/hydrophobic balance controls the nanoparticles location. Transmission electron microscopy (TEM_ shows that hphi TiO2 preferentially locates inside the PET droplets and hpho at both the interface and PP matrix. HphoM also locates within the PP matrix and at the interface, but large loadings (12%) can completely cover the surfaces of the droplets forming a physical barrier that avoids coalescence, leading to the formation of smaller droplets. A good correlation is found between the crystallization rate of PET (determined by DSC) and nanoparticles location, where hphi TiO2 induces the highest PET crystallization rate. PET lamellar morphology (revealed by TEM) is also dependent on particle location. The mechanical behavior improves in the elastic regime with TiO2 addition, but the plastic deformation of the material is limited and strongly depends on the type of TiO2 employed.
Keywords: PBNANO; blend; morphology; nanoparticles; titanium dioxide.
Conflict of interest statement
The authors declare no conflict of interest
Figures










References
-
- Plastics—The Facts 2018. [(accessed on 10 June 2019)]; Available online: https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics....
-
- Zhang Z., Wang C., Mai K. Reinforcement of recycled PET for mechanical properties of isotactic polypropylene. Adv. Ind. Eng. Polym. Res. 2019;2:69–76. doi: 10.1021/acs.iecr.8b04545. - DOI
-
- Sangroniz L., Ruiz J.L., Sangroniz A., Fernández M., Etxeberria A., Müller A.J., Santamaría A. Polyethylene terephthalate/low density polyethylene/titanium dioxide blend nanocomposites: Morphology, crystallinity, rheology, and transport properties. J. Appl. Polym. Sci. 2019;136:46986. doi: 10.1002/app.46986. - DOI
-
- Zander N.E., Gillan M., Burckhard Z., Gardea F. Recycled polypropylene blends as novel 3D printing materials. Addit. Manuf. 2019;25:122–130. doi: 10.1016/j.addma.2018.11.009. - DOI
-
- Sangroniz L., Palacios J.P., Fernández M., Eguiazabal J.I., Santamaría A., Müller A.J. Linear and non-linear rheological behaviour of polypropylene/polyamide blends modified with a compatibilizer agent and nanosilica and its relationship with the morphology. Eur. Polym. J. 2016;83:10–21. doi: 10.1016/j.eurpolymj.2016.07.026. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

