Medical, Technical and Audiological Outcomes of Hearing Rehabilitation with the Bonebridge Transcutaneous Bone-Conduction Implant: A Single-Center Experience
- PMID: 31623414
- PMCID: PMC6832994
- DOI: 10.3390/jcm8101614
Medical, Technical and Audiological Outcomes of Hearing Rehabilitation with the Bonebridge Transcutaneous Bone-Conduction Implant: A Single-Center Experience
Abstract
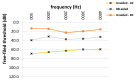
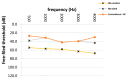
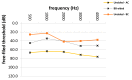
Bone-conduction implants are a standard therapeutic option for patients with conductive, unilateral, or mixed hearing loss who either do not tolerate conventional hearing aids or can benefit from surgery. The aim of this study was to evaluate long-term medical and technical outcomes, and audiological results with the Bonebridge transcutaneous bone-conduction implant. This retrospective study included all patients implanted with a bone-conduction hearing implant at a tertiary medical referral center between March 2012 and October 2018. Medical and technical outcomes included the mean length of implant usage, medical and technical complications (skin and wound infection, lack of benefit, technical failure), explantations and revisions, coupling approaches, implant failure rate, implant survival and the implant loss for added follow-up years. Auditory results were measured by functional hearing gain and the Freiburger monosyllabic test at 65 dB sound pressure level. Sixty-four patients were included in the study; five of these were implanted bilaterally (69 devices). Five unilaterally implanted patients were lost to follow-up. The mean follow-up was 27.1 months (range: 0.2 months-6.3 years). The mean implant usage was 25.9 months (range: 0.2 months-6.3 years). Fifty-seven implants (89.1%) were in use at the end of the follow-up period. Complications occurred in six ears (9.4%). Five implants (7.8%) were explanted without reimplantation. Device failure occurred in one implant (1.6%), which was possibly caused by recurrent head trauma. The rate of implant loss due to technical device failure (damage to device) was 1 per 72 follow-up years. The mean improvement on the Freiburger monosyllabic test (52.1%, p = 0.0001), and in functional hearing gain across frequencies (26.5 dB, p = 0.0001) was significant. This single-center follow-up reveals the medical and technical reliability of a transcutaneous bone-conduction implant for hearing rehabilitation because complication and revision rates were low. The majority of patients still used the device at the end of the observation period. Implantation resulted in favorable hearing outcomes in comparison to that of unaided conditions. Cautious patient selection mainly regarding co-morbidities, the history of chronic otologic diseases and proper surgical technique seems to be crucial in reducing complications.
Keywords: Bonebridge; audiological outcomes; bone conduction; complications; transcutaneous hearing implant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Schmerber S., Deguine O., Marx M., Van de Heyning P., Sterkers O., Mosnier I., Garin P., Godey B., Vincent C., Venail F., et al. Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur. Arch. Otorhinolaryngol. 2017;274:1835–1851. doi: 10.1007/s00405-016-4228-6. - DOI - PubMed
-
- Reyes R.A., Tjellstrom A., Granstrom G. Evaluation of implant losses and skin reactions around extraoral bone-anchored implants: A 0- to 8-year follow-up. Otolaryngol. Head Neck Surg. 2000;122:272–276. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

