BIIB093 (IV glibenclamide): an investigational compound for the prevention and treatment of severe cerebral edema
- PMID: 31623469
- PMCID: PMC6893098
- DOI: 10.1080/13543784.2019.1681967
BIIB093 (IV glibenclamide): an investigational compound for the prevention and treatment of severe cerebral edema
Abstract
Introduction: Brain swelling due to edema formation is a major cause of neurological deterioration and death in patients with large hemispheric infarction (LHI) and severe traumatic brain injury (TBI), especially contusion-TBI. Preclinical studies have shown that SUR1-TRPM4 channels play a critical role in edema formation and brain swelling in LHI and TBI. Glibenclamide, a sulfonylurea drug and potent inhibitor of SUR1-TRPM4, was reformulated for intravenous injection, known as BIIB093.Areas covered: We discuss the findings from Phase 2 clinical trials of BIIB093 in patients with LHI (GAMES-Pilot and GAMES-RP) and from a small Phase 2 clinical trial in patients with TBI. For the GAMES trials, we review data on objective biological variables, adjudicated edema-related endpoints, functional outcomes, and mortality which, despite missing the primary endpoint, supported the initiation of a Phase 3 trial in LHI (CHARM). For the TBI trial, we review data on MRI measures of edema and the initiation of a Phase 2 trial in contusion-TBI (ASTRAL).Expert opinion: Emerging clinical data show that BIIB093 has the potential to transform our management of patients with LHI, contusion-TBI and other conditions in which swelling leads to neurological deterioration and death.
Keywords: BIIB093; Glibenclamide; SUR1-TRPM4; cerebral edema; contusion; large hemispheric infarction; stroke; traumatic brain injury.
Conflict of interest statement
Declaration of interest
WT Kimberly and KN Sheth received grants from Remedy Pharmaceuticals during the conduct of GAMES-Pilot and GAMES-RP, and also receive research grants from Biogen. JM Simard holds a US patent (7,285,574), ‘A novel non-selective cation channel in neural cells and methods for treating brain swelling.’ JM Simard is a member of the Board of Directors and holds shares in Remedy Pharmaceuticals and is a paid consultant for Biogen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
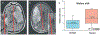
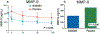


References
-
- Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology. 1998;50(2):341–350. - PubMed
-
- Hacke W, Schwab S, Horn M, et al. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53(4):309–315. - PubMed
-
- Kasner SE, Demchuk AM, Berrouschot J, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001;32(9):2117–2123. - PubMed
-
- Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76(6 Suppl):S85–90. - PubMed
-
- Iaccarino C, Carretta A, Nicolosi F, et al. Epidemiology of severe traumatic brain injury. J Neurosurg Sci. 2018;62(5):535–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS105503/NS/NINDS NIH HHS/United States
- R01 NR018335/NR/NINR NIH HHS/United States
- U24 NS107136/NS/NINDS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 NS099209/NS/NINDS NIH HHS/United States
- R01 NS105633/NS/NINDS NIH HHS/United States
- R01 HL082517/HL/NHLBI NIH HHS/United States
- I01 BX002889/BX/BLRD VA/United States
- R03 NS112859/NS/NINDS NIH HHS/United States
- U24 NS107215/NS/NINDS NIH HHS/United States
- U01 NS106513/NS/NINDS NIH HHS/United States
- R01 NS060801/NS/NINDS NIH HHS/United States
- R01 NS102589/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
