Light - driven deracemization enabled by excited - state electron transfer
- PMID: 31624212
- PMCID: PMC6939311
- DOI: 10.1126/science.aay2204
Light - driven deracemization enabled by excited - state electron transfer
Abstract
Deracemization is an attractive strategy for asymmetric synthesis, but intrinsic energetic challenges have limited its development. Here, we report a deracemization method in which amine derivatives undergo spontaneous optical enrichment upon exposure to visible light in the presence of three distinct molecular catalysts. Initiated by an excited-state iridium chromophore, this reaction proceeds through a sequence of favorable electron, proton, and hydrogen-atom transfer steps that serve to break and reform a stereogenic C-H bond. The enantioselectivity in these reactions is jointly determined by two independent stereoselective steps that occur in sequence within the catalytic cycle, giving rise to a composite selectivity that is higher than that of either step individually. These reactions represent a distinct approach to creating out-of-equilibrium product distributions between substrate enantiomers using excited-state redox events.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
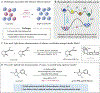
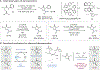


Comment in
-
Photocatalytic deracemization fixes the mix.Science. 2019 Oct 18;366(6463):304-305. doi: 10.1126/science.aay6919. Science. 2019. PMID: 31624197 No abstract available.
References
-
- Comprehensive Asymmetric Catalysis. Vol. I —III Edited by Jacobsen EN, Pfaltz A, and Yamamoto H. Springer-Verlag, Berlin: (1999).
-
- Carnell AJ, Biotransformations, 57–72 (2001).
-
- Servi S, Tessaro D, Pedrocchi-Fantoni G. Coord. Chem Rev 252, 715–726 (2008).
-
- Kroutil W, Faber K, Tetrahedron: Asymmetry, 9, 2901–2913 (1998).
-
- Blackmond DG. Angewandte Chemie Int Ed. 48, 2648–2654 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

