Boron: Its Role in Energy-Related Processes and Applications
- PMID: 31625661
- PMCID: PMC7317435
- DOI: 10.1002/anie.201911108
Boron: Its Role in Energy-Related Processes and Applications
Abstract
Boron's unique position in the Periodic Table, that is, at the apex of the line separating metals and nonmetals, makes it highly versatile in chemical reactions and applications. Contemporary demand for renewable and clean energy as well as energy-efficient products has seen boron playing key roles in energy-related research, such as 1) activating and synthesizing energy-rich small molecules, 2) storing chemical and electrical energy, and 3) converting electrical energy into light. These applications are fundamentally associated with boron's unique characteristics, such as its electron-deficiency and the availability of an unoccupied p orbital, which allow the formation of a myriad of compounds with a wide range of chemical and physical properties. For example, boron's ability to achieve a full octet of electrons with four covalent bonds and a negative charge has led to the synthesis of a wide variety of borate anions of high chemical and electrochemical stability-in particular, weakly coordinating anions. This Review summarizes recent advances in the study of boron compounds for energy-related processes and applications.
Keywords: OLEDs; boron; electrolytes; hydrogen; small-molecule activation.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





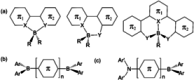
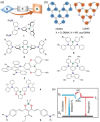
References
-
- None
-
- Wang B., Li Y., Ganguly R., Webster R. D., Kinjo R., Angew. Chem. Int. Ed. 2018, 57, 7826–7829; - PubMed
- Angew. Chem. 2018, 130, 7952–7955;
-
- Stennett T. E., Bissinger P., Griesbeck S., Ullrich S., Krummenacher I., Auth M., Sperlich A., Stolte M., Radacki K., Yao C.-J., Würthner F., Steffen A., Marder T. B., Braunschweig H., Angew. Chem. Int. Ed. 2019, 58, 6449–6454; - PubMed
- Angew. Chem. 2019, 131, 6516–6521.
-
- None
-
- Segawa Y., Yamashita M., Nozaki K., Science 2006, 314, 113–115; - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

