Deletion of the microRNA-degrading nuclease, translin/trax, prevents pathogenic vascular stiffness
- PMID: 31625778
- PMCID: PMC6879913
- DOI: 10.1152/ajpheart.00153.2019
Deletion of the microRNA-degrading nuclease, translin/trax, prevents pathogenic vascular stiffness
Abstract
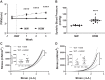
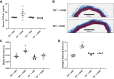
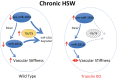
Vascular stiffness plays a key role in the pathogenesis of hypertension. Recent studies indicate that the age-associated reduction in miR-181b levels in vascular smooth muscle cells (VSMCs) contributes to increased vascular stiffness. As these findings suggest that inhibiting degradation of miR-181b might prevent vascular stiffening, we have assessed whether the microRNA-degrading translin/trax (TN/TX) complex mediates degradation of miR-181b in the aorta.We found that TN-/- mice display elevated levels of miR-181b expression in the aorta. Therefore, we tested whether TN deletion prevents vascular stiffening in a mouse model of hypertension, induced by chronic high-salt intake (4%NaCl in drinking water for 3 wk; HSW). TN-/- mice subjected to HSW stress do not show increased vascular stiffness, as monitored by pulse wave velocity and tensile testing. The protective effect of TN deletion in the HSW paradigm appears to be mediated by its ability to increase miR-181b in the aorta since HSW decreases levels of miR-181b in WT mice, but not in TN KO mice. We demonstrate for the first time that interfering with microRNA degradation can have a beneficial impact on the vascular system and identify the microRNA-degrading TN/TX RNase complex as a potential therapeutic target in combatting vascular stiffness.NEW & NOTEWORTHY While the biogenesis and mechanism of action of mature microRNA are well understood, much less is known about the regulation of microRNA via degradation. Recent studies have identified the protein complex, translin(TN)/trax(TX), as a microRNA-degrading enzyme. Here, we demonstrate that TN/TX is expressed in vascular smooth muscle cells. Additionally, deletion of the TN/TX complex selectively increases aortic miR-181b and prevents increased vascular stiffness caused by ingestion of high-salt water. To our knowledge, this is first report describing the role of a microRNA RNAse in cardiovascular biology or pathobiology.
Keywords: hypertension; miR-181b; miRNA-degradation, translin/trax complex, vascular stiffness.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

