Distinctive microbial communities in subzero hypersaline brines from Arctic coastal sea ice and rarely sampled cryopegs
- PMID: 31626297
- PMCID: PMC6859516
- DOI: 10.1093/femsec/fiz166
Distinctive microbial communities in subzero hypersaline brines from Arctic coastal sea ice and rarely sampled cryopegs
Abstract
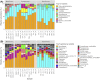
Hypersaline aqueous environments at subzero temperatures are known to be inhabited by microorganisms, yet information on community structure in subzero brines is very limited. Near Utqiaġvik, Alaska, we sampled subzero brines (-6°C, 115-140 ppt) from cryopegs, i.e. unfrozen sediments within permafrost that contain relic (late Pleistocene) seawater brine, as well as nearby sea-ice brines to examine microbial community composition and diversity using 16S rRNA gene amplicon sequencing. We also quantified the communities microscopically and assessed environmental parameters as possible determinants of community structure. The cryopeg brines harbored surprisingly dense bacterial communities (up to 108 cells mL-1) and millimolar levels of dissolved and particulate organic matter, extracellular polysaccharides and ammonia. Community composition and diversity differed between the two brine environments by alpha- and beta-diversity indices, with cryopeg brine communities appearing less diverse and dominated by one strain of the genus Marinobacter, also detected in other cold, hypersaline environments, including sea ice. The higher density and trend toward lower diversity in the cryopeg communities suggest that long-term stability and other features of a subzero brine are more important selective forces than in situ temperature or salinity, even when the latter are extreme.
Keywords: Marinobacter; Arctic; bacterial diversity; cryopeg; sea ice; subsurface microbiology.
© FEMS 2019.
Figures




References
-
- Boetius A, Anesio AM, Deming JW et al. .. Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat Rev Microbiol. 2015;13:677–90. - PubMed
-
- Bowman JS. The relationship between sea ice bacterial community structure and biogeochemistry: a synthesis of current knowledge and known unknowns. Elem Sci Anth. 2015;3:000072.
-
- Brown MV, Bowman JP. A molecular phylogenetic survey of sea-ice microbial communities (SIMCO). FEMS Microbiol Ecol. 2001;35:267–75. - PubMed

