Determination of Ligand Profiles for Pseudomonas aeruginosa Solute Binding Proteins
- PMID: 31627455
- PMCID: PMC6829864
- DOI: 10.3390/ijms20205156
Determination of Ligand Profiles for Pseudomonas aeruginosa Solute Binding Proteins
Abstract
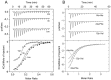
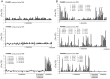
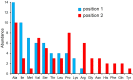
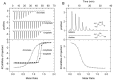
Solute binding proteins (SBPs) form a heterogeneous protein family that is found in all kingdoms of life. In bacteria, the ligand-loaded forms bind to transmembrane transporters providing the substrate. We present here the SBP repertoire of Pseudomonas aeruginosa PAO1 that is composed of 98 proteins. Bioinformatic predictions indicate that many of these proteins have a redundant ligand profile such as 27 SBPs for proteinogenic amino acids, 13 proteins for spermidine/putrescine, or 9 proteins for quaternary amines. To assess the precision of these bioinformatic predictions, we have purified 17 SBPs that were subsequently submitted to high-throughput ligand screening approaches followed by isothermal titration calorimetry studies, resulting in the identification of ligands for 15 of them. Experimentation revealed that PA0222 was specific for γ-aminobutyrate (GABA), DppA2 for tripeptides, DppA3 for dipeptides, CysP for thiosulphate, OpuCC for betaine, and AotJ for arginine. Furthermore, RbsB bound D-ribose and D-allose, ModA bound molybdate, tungstate, and chromate, whereas AatJ recognized aspartate and glutamate. The majority of experimentally identified ligands were found to be chemoattractants. Data show that the ligand class recognized by SPBs can be predicted with confidence using bioinformatic methods, but experimental work is necessary to identify the precise ligand profile.
Keywords: chemotaxis; ligand recognition; solute binding protein; transport.
Conflict of interest statement
The authors do not declare any conflict of interest.
Figures

References
-
- Whitchurch C.B., Leech A.J., Young M.D., Kennedy D., Sargent J.L., Bertrand J.J., Semmler A.B., Mellick A.S., Martin P.R., Alm R.A., et al. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol. Microbiol. 2004;52:873–893. doi: 10.1111/j.1365-2958.2004.04026.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

