Analysis of spontaneous reporting of suspected adverse drug reactions for non-analgesic over-the-counter drugs from 2008 to 2017
- PMID: 31627740
- PMCID: PMC6798506
- DOI: 10.1186/s40360-019-0338-2
Analysis of spontaneous reporting of suspected adverse drug reactions for non-analgesic over-the-counter drugs from 2008 to 2017
Abstract
Background: Adverse drug reaction (ADR) reporting practices by health care professionals remain poor. Over-the-counter (OTC) drugs are perceived as safe; however, they can also cause ADRs. The objective of this study was to analyze ADR reporting for OTC drugs in a 10-year period, in order to evaluate frequency of ADRs, population that ADRs most affect and reporters of ADRs of OTC drugs in Croatia.
Methods: Spontaneously reported ADRs of non-analgesic OTC drugs, collected from January 2008 to December 2017 were analyzed. Data was obtained from Agency for Medicinal Products and Medical Devices of Croatia (HALMED).
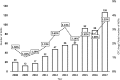
Results: There were 547 ADRs of OTC drugs reported in total and an increase in number of reports through the years was observed. Pharmacists reported 45.4% of all ADRs, and were most frequent reporters (p < 0.001). In 2017 majority of reports, 62 (49.2%), were obtained from consumers. ADRs were most frequently observed in patients aged 70 years and older (15% of ADRs). Five percent of all reports were accidental exposures among children.
Conclusions: Pharmacists most frequently reported ADRs of OTC drugs and consumers' awareness of ADR reporting has risen. Other health care professionals (e.g., nurses and dentists) must be offered proper education in order to improve reporting practice of ADRs. Health care professionals should address concerns about OTC drug safety in elderly and children.
Keywords: Adverse drug reactions; Drug safety; OTC drugs; Pharmacovigilance; Spontaneous reporting.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- World Health Organization. http://apps.who.int/medicinedocs/pdf/whozip32e/whozip32e.pdf. Accessed 27 November 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials