Mutations in thyroid hormone receptor α1 cause premature neurogenesis and progenitor cell depletion in human cortical development
- PMID: 31628250
- PMCID: PMC6842615
- DOI: 10.1073/pnas.1908762116
Mutations in thyroid hormone receptor α1 cause premature neurogenesis and progenitor cell depletion in human cortical development
Erratum in
-
Correction for Krieger et al., Mutations in thyroid hormone receptor α1 cause premature neurogenesis and progenitor cell depletion in human cortical development.Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7537-7538. doi: 10.1073/pnas.2003767117. Epub 2020 Mar 23. Proc Natl Acad Sci U S A. 2020. PMID: 32205435 Free PMC article. No abstract available.
Abstract
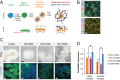
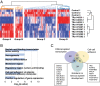
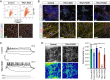
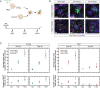
Mutations in the thyroid hormone receptor α 1 gene (THRA) have recently been identified as a cause of intellectual deficit in humans. Patients present with structural abnormalities including microencephaly, reduced cerebellar volume and decreased axonal density. Here, we show that directed differentiation of THRA mutant patient-derived induced pluripotent stem cells to forebrain neural progenitors is markedly reduced, but mutant progenitor cells can generate deep and upper cortical layer neurons and form functional neuronal networks. Quantitative lineage tracing shows that THRA mutation-containing progenitor cells exit the cell cycle prematurely, resulting in reduced clonal output. Using a micropatterned chip assay, we find that spatial self-organization of mutation-containing progenitor cells in vitro is impaired, consistent with down-regulated expression of cell-cell adhesion genes. These results reveal that thyroid hormone receptor α1 is required for normal neural progenitor cell proliferation in human cerebral cortical development. They also exemplify quantitative approaches for studying neurodevelopmental disorders using patient-derived cells in vitro.
Keywords: brain development; iPSCs; thyroid hormone.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Bath S. C., Steer C. D., Golding J., Emmett P., Rayman M. P., Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382, 331–337 (2013). - PubMed
-
- Mohan V., et al. , Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp. Neurol. 237, 477–488 (2012). - PubMed
-
- Pathak A., Sinha R. A., Mohan V., Mitra K., Godbole M. M., Maternal thyroid hormone before the onset of fetal thyroid function regulates reelin and downstream signaling cascade affecting neocortical neuronal migration. Cereb. Cortex 21, 11–21 (2011). - PubMed
-
- Ausó E., et al. , A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145, 4037–4047 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G1002276/MRC_/Medical Research Council/United Kingdom
- MC_UU_00014/5/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- G0300117/MRC_/Medical Research Council/United Kingdom
- G0600717/MRC_/Medical Research Council/United Kingdom
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- CRUK_/Cancer Research UK/United Kingdom
- MC_PC_12009/MRC_/Medical Research Council/United Kingdom
- 210755/Z/18/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

