The importance of Antarctic krill in biogeochemical cycles
- PMID: 31628346
- PMCID: PMC6800442
- DOI: 10.1038/s41467-019-12668-7
The importance of Antarctic krill in biogeochemical cycles
Erratum in
-
Author Correction: The importance of Antarctic krill in biogeochemical cycles.Nat Commun. 2019 Nov 20;10(1):5340. doi: 10.1038/s41467-019-13390-0. Nat Commun. 2019. PMID: 31745095 Free PMC article.
Abstract
Antarctic krill (Euphausia superba) are swarming, oceanic crustaceans, up to two inches long, and best known as prey for whales and penguins - but they have another important role. With their large size, high biomass and daily vertical migrations they transport and transform essential nutrients, stimulate primary productivity and influence the carbon sink. Antarctic krill are also fished by the Southern Ocean's largest fishery. Yet how krill fishing impacts nutrient fertilisation and the carbon sink in the Southern Ocean is poorly understood. Our synthesis shows fishery management should consider the influential biogeochemical role of both adult and larval Antarctic krill.
Conflict of interest statement
Steve Nicol has been employed to provide scientific advice to the Association of Responsible Krill harvesting companies.
Figures
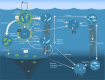


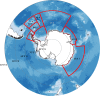

References
-
- Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–206. - PubMed
-
- Pauly D, Christensen V. Primary production required to sustain global fisheries. Nature. 1995;374:255.
-
- Falkowski PG. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994;39:235–258. - PubMed
-
- Robinson, C. Phytoplankton biogeochemical cycles. in Marine Plankton: A practical guide to ecology, methodology, and taxonomy (Castellani, C. & Edwards, M. eds.) (OUP, 2017).
-
- Steinberg DK, et al. Bacterial vs. zooplankton control of sinking particle flux in the ocean’s twilight zone. Limnol. Oceanogr. 2008;53:1327–1338.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

