Impact of Early Life Antibiotic Exposure and Neonatal Hyperoxia on the Murine Microbiome and Lung Injury
- PMID: 31628395
- PMCID: PMC6802223
- DOI: 10.1038/s41598-019-51506-0
Impact of Early Life Antibiotic Exposure and Neonatal Hyperoxia on the Murine Microbiome and Lung Injury
Abstract
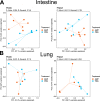
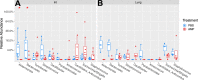
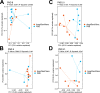
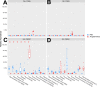
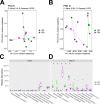
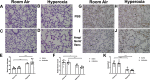
Cross talk between the intestinal microbiome and the lung and its role in lung health remains unknown. Perinatal exposure to antibiotics disrupts the neonatal microbiome and may have an impact on the preterm lung. We hypothesized that perinatal antibiotic exposure leads to long-term intestinal dysbiosis and increased alveolar simplification in a murine hyperoxia model. Pregnant C57BL/6 wild type dams and neonatal mice were treated with antibiotics before and/or immediately after delivery. Control mice received phosphate-buffered saline (PBS). Neonatal mice were exposed to 95% oxygen for 4 days or room air. Microbiome analysis was performed using 16S rRNA gene sequencing. Pulmonary alveolarization and vascularization were analyzed at postnatal day (PND) 21. Perinatal antibiotic exposure modified intestinal beta diversity but not alpha diversity in neonatal mice. Neonatal hyperoxia exposure altered intestinal beta diversity and relative abundance of commensal bacteria in antibiotic treated mice. Hyperoxia disrupted pulmonary alveolarization and vascularization at PND 21; however, there were no differences in the degree of lung injury in antibiotic treated mice compared to vehicle treated controls. Our study suggests that exposure to both hyperoxia and antibiotics early in life may cause long-term alterations in the intestinal microbiome, but intestinal dysbiosis may not significantly influence neonatal hyperoxic lung injury.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- R56 ES030221/ES/NIEHS NIH HHS/United States
- R01 HL129794/HL/NHLBI NIH HHS/United States
- P30 ES030285/ES/NIEHS NIH HHS/United States
- 1R56ES030221/U.S. Department of Health & Human Services | NIH | National Institute of Environmental Health Sciences (NIEHS)/International
- K08 HL127103/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

