Physiologically-Based Pharmacokinetic Models for Evaluating Membrane Transporter Mediated Drug-Drug Interactions: Current Capabilities, Case Studies, Future Opportunities, and Recommendations
- PMID: 31628859
- PMCID: PMC7232864
- DOI: 10.1002/cpt.1693
Physiologically-Based Pharmacokinetic Models for Evaluating Membrane Transporter Mediated Drug-Drug Interactions: Current Capabilities, Case Studies, Future Opportunities, and Recommendations
Abstract
Physiologically-based pharmacokinetic (PBPK) modeling has been extensively used to quantitatively translate in vitro data and evaluate temporal effects from drug-drug interactions (DDIs), arising due to reversible enzyme and transporter inhibition, irreversible time-dependent inhibition, enzyme induction, and/or suppression. PBPK modeling has now gained reasonable acceptance with the regulatory authorities for the cytochrome-P450-mediated DDIs and is routinely used. However, the application of PBPK for transporter-mediated DDIs (tDDI) in drug development is relatively uncommon. Because the predictive performance of PBPK models for tDDI is not well established, here, we represent and discuss examples of PBPK analyses included in regulatory submission (the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the Pharmaceuticals and Medical Devices Agency (PMDA)) across various tDDIs. The goal of this collaborative effort (involving scientists representing 17 pharmaceutical companies in the Consortium and from academia) is to reflect on the use of current databases and models to address tDDIs. This challenges the common perceptions on applications of PBPK for tDDIs and further delves into the requirements to improve such PBPK predictions. This review provides a reflection on the current trends in PBPK modeling for tDDIs and provides a framework to promote continuous use, verification, and improvement in industrialization of the transporter PBPK modeling.
© 2019 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Howard Burt and Sibylle Neuhoff are employees of Simcyp Limited (a Certara Company). Yuichi Sugiyama is the member of the Simcyp Limited (a Certara Company) Scientific Advisory Board. The other authors have no conflicts of interest to declare.
Figures

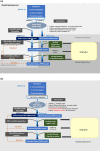

References
-
- European Medical Agency. Guideline on the investigation of drug interactions (European Medical Agency, London, 2012).
-
- US Food and Drug Administration . In Vitro Metabolism‐ and Transporter‐Mediated Drug‐Drug Interaction Studies – Guidance for Industry (Draft Guidance) (2017).
-
- Pan, Y. et al The application of physiologically based pharmacokinetic modeling to predict the role of drug transporters: scientific and regulatory perspectives. J. Clin. Pharmacol. 56, S122–S131 (2016). - PubMed
-
- Varma, M.V. , Steyn, S.J. , Allerton, C. & El‐Kattan, A.F. Predicting clearance mechanism in drug discovery: extended clearance classification system (ECCS). Pharm. Res. 32, 3785–3802 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

