Cardiomyocytes from CCND2-overexpressing human induced-pluripotent stem cells repopulate the myocardial scar in mice: A 6-month study
- PMID: 31629738
- PMCID: PMC7346870
- DOI: 10.1016/j.yjmcc.2019.09.011
Cardiomyocytes from CCND2-overexpressing human induced-pluripotent stem cells repopulate the myocardial scar in mice: A 6-month study
Abstract
Background: Cardiomyocytes that have been differentiated from CCND2-overexpressing human induced-pluripotent stem cells (hiPSC-CCND2OE CMs) can proliferate when transplanted into mouse hearts after myocardial infarction (MI). However, it is unknown whether remuscularization can replace the thin LV scar and if the large muscle graft can electrophysiologically synchronize to the recipient myocardium. Our objectives are to evaluate the structural and functional potential of hiPSC-CCND2OE CMs in replacing the LV thin scar.
Methods: NOD/SCID mice were treated with hiPSC-CCND2OE CMs (i.e., the CCND2OE group), hiPSC-CCND2WT CMs (the CCND2WT group), or an equal volume of PBS immediately after experimentally-induced myocardial infarction. The treatments were administered to one site in the infarcted zone (IZ), two sites in the border zone (BZ), and a fourth group of animals underwent Sham surgery.
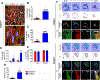
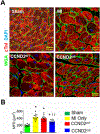
Results: Six months later, engrafted cells occupied >50% of the scarred region in CCND2OE animals, and exceeded the number of engrafted cells in CCND2WT animals by ~8-fold. Engrafted cells were also more common in the IZ than in the BZ for both cell-treatment groups. Measurements of cardiac function, infarct size, wall thickness, and cardiomyocyte hypertrophy were significantly improved in CCND2OE animals compared to animals from the CCND2WT or PBS-treatment groups. Measurements in the CCND2WT and PBS groups were similar, and markers for cell cycle activation and proliferation were significantly higher in hiPSC-CCND2OE CMs than in hiPSC-CCND2WT CMs. Optical mapping of action potential propagation indicated that the engrafted hiPSC-CCND2OE CMs were electrically coupled to each other and to the cells of the native myocardium. No evidence of tumor formation was observed in any animals.
Conclusions: Six months after the transplantation, CCND2-overexpressing hiPSC-CMs proliferated and replaced >50% of the myocardial scar tissue. The large graft hiPSC-CCND2OE CMs also electrically integrated with the host myocardium, which was accompanied by a significant improvement in LV function.
Keywords: Cell cycle; Heart failure; Induced pluripotent stem cells; Myocardial infarction.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures
The authors have declared that no conflict of interest exists.
Figures





References
-
- van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, et al., Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction, Stem. Cell. Res. 1 (2007) 9–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

