Identifying regulatory pathways of spleen tyrosine kinase expression in human basophils
- PMID: 31629804
- PMCID: PMC7062573
- DOI: 10.1016/j.jaci.2019.10.005
Identifying regulatory pathways of spleen tyrosine kinase expression in human basophils
Abstract
Background: Expression levels of spleen tyrosine kinase (SYK), a critical signaling tyrosine kinase in basophils, are uniquely low relative to all other circulating leukocytes, and levels are highly variable in the population.
Objective: We sought to determine whether transcriptional regulation of SYK through unique silencing of the SYK gene determines its basophil-specific expression patterns.
Methods: Culture-derived basophils (CD34B cells) were derived from cultures of CD34+ progenitor cells by using 2 methods (G1 or G3). Peripheral blood basophils (PBBs; relative SYK protein level = 1), B cells (SYK = 8), CD34B-G1 cells (SYK = 11), and CD34B-G3 cells (SYK = 5) were examined by using assay for transposase-accessible chromatin sequencing (ATAC-seq) methods. In addition, the transcriptomes of 6 cell types, PBBs, peripheral blood eosinophils (SYK = 11), plasmacytoid dendritic cells (SYK = 30), CD34+ progenitors (SYK = 11), CD34B-G1 cells, and CD34B-G3 cells, were analyzed for patterns that matched patterns of SYK expression in these cells, with a focus on transcription factors.
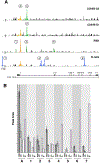
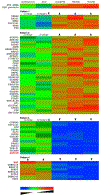
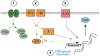
Results: ATAC-seq showed that PBBs have multiple open regions in the SYK gene, suggesting a nonsilenced state with 1 region unique to PBBs (low SYK expression), 1 region unique to both PBBs (low SYK expression) and both G1 and G3 CD34B cells (high and moderate SYK expression, respectively), and 5 regions unique to B cells (high SYK expression). SYK expression across the 6 cell types explored showed a unique pattern that was matched to expression patterns of 3 transcription factors: Kruppel-like factor 5 (KLF5), zinc-finger protein 608 (ZNF608), and musculoaponeurotic fibrosarcoma protein (c-MAF).
Conclusions: Two new potential regulatory pathways for SYK expression were identified. One appears independent of transcriptional regulation, and one appears to be dependent on transcriptional control in the SYK gene.
Keywords: Human; allergy; basophil; development; signal transduction.
Copyright © 2019 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
COI: All authors have no conflicts of interest relevant to this manuscript to report.
Figures


References
-
- Vilarino N, MacGlashan D Jr. Transient transfection of human peripheral blood basophils. J Immunol Methods 2005; 296:11–8. - PubMed
-
- MacGlashan DW Jr., Undem BJ. Inducing an Anergic State in Mast Cells and Basophils without Secretion. J. Allergy Clin. Immunol 2008; 121:1500–6. - PubMed
-
- Havard S, Scola AM, Kay LJ, Ishmael SS, Macglashan DW Jr., Peachell PT. Characterization of syk expression in human lung mast cells: relationship with function. Clin Exp Allergy 2011; 41:378–88. - PubMed
-
- MacGlashan DW Jr. Relationship Between Syk and SHIP Expression and Secretion from Human Basophils in the General Population. J. Allergy Clin. Immunol 2007; 119:626–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

