Understanding Treatment Preferences in Patients with Moderate to Severe Plaque Psoriasis in the USA: Results from a Cross-Sectional Patient Survey
- PMID: 31630336
- PMCID: PMC6828866
- DOI: 10.1007/s13555-019-00334-1
Understanding Treatment Preferences in Patients with Moderate to Severe Plaque Psoriasis in the USA: Results from a Cross-Sectional Patient Survey
Abstract
Introduction: The goal of psoriasis (PsO) treatment is to improve quality of life by lessening the extent and severity of the disease. Traditional systemic drugs and biologic agents are used for the treatment of moderate to severe PsO and recent research emphasizes understanding patient goals and preferences for treatment, to improve overall outcomes.
Methods: An online survey was administered to collect data from 500 adult patients with moderate to severe PsO in the USA. Patients were required to have current or previous systemic therapy use and were excluded if aged 75 or older. Data on demographics, disease burden, treatment use, and patients' treatment goals and expectations were collected. Descriptive and multivariate analyses examined the factors that predict treatment goals. Subgroup analyses were performed for age, gender, severity, comorbid psoriatic arthritis (PsA), location of PsO, and biologic experience. All analyses were conducted using SAS v9.4 and R v3.4.
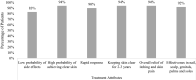
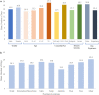
Results: Of the 500 adult patients included, 71.6% reported moderate PsO. Patients had a mean (SD) score of 62.4 (23.0) for skin pain, 60.0 (26.3) for fatigue, and 6.6 (2.1) for itch on a scale of 0-100, 0-100, and 0-10 respectively. Mean (SD) score for quality of life (QoL), assessed using Dermatology Life Quality Index (DLQI), was 18.3 (7.3), with more than 90% having moderate/very large/extremely large effect on life. The majority of patients considered "keeping skin clear for 2-3 years" (94%), "overall relief of symptoms" (93.8%), and effective in clearing certain areas" (92.2%) as important attributes of a systemic treatment. Overall, patients expected 50% clear skin in about 2 weeks and completely clear skin in about 4 weeks.
Conclusions: Overall, in this study with more than 70% of patients with moderate disease, patients reported high burden of disease and impact on QoL. This study demonstrates the importance of considering patient perspectives in treatment decisions that are critical for optimizing patient outcomes.
Funding: Eli Lilly and Company.
Keywords: Patient-reported outcomes; Plaque psoriasis; Rapid response; Real-world analysis; Treatment expectations; Treatment goals.
Conflict of interest statement
Joe Gorelick has had a financial agreement or affiliation with the following commercial interests as a consultant for AbbVie Pharmaceuticals, Dermira, Lilly, LEO, Novartis Pharmaceuticals, Ortho Dermatologics, Pfizer, PruGen, Regeneron Pharmaceuticals, Sanofi Genzyme, Sun Pharma, and UCB; served on Advisory Board/Speaker’s Bureau for AbbVie Pharmaceuticals, Dermira, Lilly, LEO Pharma, Novartis Pharmaceuticals, Ortho Dermatologics, Pfizer, PruGen, Regeneron Pharmaceuticals, Sun Pharma, and UCB. David Rosmarin has received honoraria as a consultant for AbbVie, Celgene, Dermavant, Dermira, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi; has received research support from AbbVie, Bristol Meyers Squibb, Celgene, Dermira, Incyte, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron Pharmaceuticals Inc; and has served as a paid speaker for AbbVie, Celgene, Janssen, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi. David Shrom is a current employee and shareholder of Eli Lilly and Company. Kiran Sikand is a current employee and shareholder of Eli Lilly and Company. Lisa Renda is a current employee and shareholder of Eli Lilly and Company. Russel Burge is a current employee and shareholder of Eli Lilly and Company. Craig Krebsbach is a current employee of Ipsos Insights LLC. Christine Dworkin was an employee of Ipsos Insights LLC at the time of the study. Chitra Karki was an employee of Ipsos Insights LLC at the time of the study. Ripsi P. Patel was an employee of Ipsos Insights LLC at the time of the study.
Figures

References
-
- Feldman SR, Regnier SA, Chirilov A, Hey F, Gilloteau I, Cella D. Patient-reported outcomes are important elements of psoriasis treatment decision making: a discrete choice experiment survey of dermatologists in the United States. J Am Acad Dermatol. 2019;80(6):150–1657. doi: 10.1016/j.jaad.2019.01.039. - DOI - PubMed
-
- Mayo Clinic. Psoriasis overview. 2019. https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes.... Accessed 17 Oct 2019.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

