Validation of a host response test to distinguish bacterial and viral respiratory infection
- PMID: 31631046
- PMCID: PMC6838360
- DOI: 10.1016/j.ebiom.2019.09.040
Validation of a host response test to distinguish bacterial and viral respiratory infection
Abstract
Background: Distinguishing bacterial and viral respiratory infections is challenging. Novel diagnostics based on differential host gene expression patterns are promising but have not been translated to a clinical platform nor extensively tested. Here, we validate a microarray-derived host response signature and explore performance in microbiology-negative and coinfection cases.
Methods: Subjects with acute respiratory illness were enrolled in participating emergency departments. Reference standard was an adjudicated diagnosis of bacterial infection, viral infection, both, or neither. An 87-transcript signature for distinguishing bacterial, viral, and noninfectious illness was measured from peripheral blood using RT-PCR. Performance characteristics were evaluated in subjects with confirmed bacterial, viral, or noninfectious illness. Subjects with bacterial-viral coinfection and microbiologically-negative suspected bacterial infection were also evaluated. Performance was compared to procalcitonin.
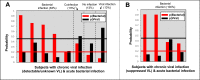
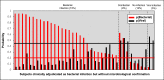
Findings: 151 subjects with microbiologically confirmed, single-etiology illness were tested, yielding AUROCs 0•85-0•89 for bacterial, viral, and noninfectious illness. Accuracy was similar to procalcitonin (88% vs 83%, p = 0•23) for bacterial vs. non-bacterial infection. Whereas procalcitonin cannot distinguish viral from non-infectious illness, the RT-PCR test had 81% accuracy in making this determination. Bacterial-viral coinfection was subdivided. Among 19 subjects with bacterial superinfection, the RT-PCR test identified 95% as bacterial, compared to 68% with procalcitonin (p = 0•13). Among 12 subjects with bacterial infection superimposed on chronic viral infection, the RT-PCR test identified 83% as bacterial, identical to procalcitonin. 39 subjects had suspected bacterial infection; the RT-PCR test identified bacterial infection more frequently than procalcitonin (82% vs 64%, p = 0•02).
Interpretation: The RT-PCR test offered similar diagnostic performance to procalcitonin in some subgroups but offered better discrimination in others such as viral vs. non-infectious illness and bacterial/viral coinfection. Gene expression-based tests could impact decision-making for acute respiratory illness as well as a growing number of other infectious and non-infectious diseases.
Keywords: Biomarkers; Coinfection; Diagnosis; Gene expression; Precision medicine; Respiratory tract infections.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
TWB reports other from Predigen Inc, outside the submitted work; In addition, TWB has a patent “METHODS TO DIAGNOSE AND TREAT ACUTE RESPIRATORY INFECTIONS” (USPTO Pub. No.: US 2018/0245154 A1) pending. CBC reports grants from National Institutes of Health, grants from DARPA, during the conduct of the study; personal fees from National Institutes of Health, outside the submitted work. VGF served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Basilea Pharmaceutica, Karius, ContraFect, Regeneron Pharmaceuticals, and Genentech; has NIH STTR/SBIR grants pending with Affinergy, Locus, and Medical Surface, Inc; has been a consultant for Achaogen, AmpliPhi Biosciences, Astellas Pharma, Arsanis, Affinergy, Basilea Pharmaceutica, Bayer, Cerexa Inc., ContraFect, Cubist, Debiopharm, Durata Therapeutics, Grifols, Genentech, MedImmune, Merck, The Medicines Company, Pfizer, Novartis, NovaDigm Therapeutics Inc., Theravance Biopharma, Inc., XBiotech, and has received honoraria from Theravance Biopharma, Inc., and Green Cross, and has a patent pending in sepsis diagnostics. GSG reports other from Predigen outside the submitted work; In addition, GSG has a patent “Biomarkers for the molecular classification of bacterial infection” US 14/1880,668 pending, and a patent “Methods of Identifying Infectious Disease and Assays for Identifying Infectious Disease” US patent US 8,821,876 issued. RH reports grants from ARLG/NIH during the conduct of the study; In addition, RH has a patent “Methods to Diagnose and Treat Acute Respiratory Infections” (Application #PCT/US2016/040437) pending. SFK reports grants from National Institutes of Health during the conduct of the study. MTM reports consultant fees from UpToDate, outside the submitted work; In addition, MTM has a patent Patent pending on Genomic Diagnostics for Respiratory Infections. EBQ reports grants from NIAID, grants from DARPA during the conduct of the study. ELT reports personal fees from Duke University, personal fees from Durham VA Health Care System, grants from DARPA, grants from NIH/ARLG, other from Predigen, Inc., grants from NIH/VTEU, personal fees from bioMerieux during the conduct of the study. In addition, DELT has a patent “Biomarkers for the molecular classification of bacterial infection” pending, and a patent “Methods to diagnose and treat acute respiratory infections” pending. CWW reports personal fees from Duke University, personal fees from Durham VA Health Care System, grants from DARPA, grants from NIH/ARLG, other from Predigen, Inc., grants from NIH/VTEU, personal fees from bioMerieux, personal fees from IDbyDNA, personal fees from Giner, grants from BioFire, grants from Janus, grants from BioMeme, grants from RTI, personal fees from Roche Molecular Sciences, during the conduct of the study; personal fees from Becton Dickinson, grants from Pfizer, grants from Openbiome, grants from MRI Global, personal fees from Sanofi, outside the submitted work; In addition, CWW has a patent “Biomarkers for the molecular classification of bacterial infection “pending, and a patent “ Methods to diagnose and treat acute respiratory infections pending”. Other authors not explicitly listed above have nothing to disclose.
Figures


References
-
- WHO. Antibacterial agents in clinical development, 2017.
-
- Odermatt J., Friedli N., Kutz A. Effects of procalcitonin testing on antibiotic use and clinical outcomes in patients with upper respiratory tract infections. An individual patient data meta-analysis. Clin Chem Lab Med. 2017 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

