Altitude and life-history shape the evolution of Heliconius wings
- PMID: 31631338
- PMCID: PMC6916360
- DOI: 10.1111/evo.13865
Altitude and life-history shape the evolution of Heliconius wings
Abstract
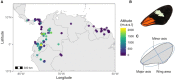
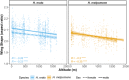
Phenotypic divergence between closely related species has long interested biologists. Taxa that inhabit a range of environments and have diverse natural histories can help understand how selection drives phenotypic divergence. In butterflies, wing color patterns have been extensively studied but diversity in wing shape and size is less well understood. Here, we assess the relative importance of phylogenetic relatedness, natural history, and habitat on shaping wing morphology in a large dataset of over 3500 individuals, representing 13 Heliconius species from across the Neotropics. We find that both larval and adult behavioral ecology correlate with patterns of wing sexual dimorphism and adult size. Species with solitary larvae have larger adult males, in contrast to gregarious Heliconius species, and indeed most Lepidoptera, where females are larger. Species in the pupal-mating clade are smaller than those in the adult-mating clade. Interestingly, we find that high-altitude species tend to have rounder wings and, in one of the two major Heliconius clades, are also bigger than their lowland relatives. Furthermore, within two widespread species, we find that high-altitude populations also have rounder wings. Thus, we reveal novel adaptive wing morphological divergence among Heliconius species beyond that imposed by natural selection on aposematic wing coloration.
Keywords: Altitude; Heliconius; Lepidoptera; phenotypic divergence; sexual dimorphism; wing morphology.
© 2019 The Author(s). Evolution published by Wiley Periodicals, Inc. on behalf of The Society for the Study of Evolution.
Figures



References
-
- Abouheif, E. 1999. A method for testing the assumption of phylogenetic independence in comparative data. Evol. Ecol. Res. 1:895–909.
-
- Allen, C. E. , Zwaan B. J., and Brakefield P. M.. 2011. Evolution of sexual dimorphism in the Lepidoptera. Annu. Rev. Entomol. 56:445–464. - PubMed
-
- Altizer, S. , and Davis A. K.. 2010. Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 64:1018–1028. - PubMed
-
- Azevedo, R. B. R. , James A. C., McCabe J., and Partridge L.. 1998. Latitudinal variation of wing: thorax size ratio and wing‐aspect ratio in Drosophila melanogaster . Evolution 52:1353–1362. - PubMed
-
- Barton, K. and Barton M. K., 2019. Package ‘MuMIn’. Multi‐model inference. version, 1(6).
Publication types
MeSH terms
Grants and funding
- NE/L002507/1/Natural Environment Research Council/International
- NE/R010331/1/Natural Environment Research Council/International
- NE/R153436/1/Natural Environment Research Council/International
- Scurfield Memorial Bursary 2017/University of Sheffield/International
- 2018SPE0000400194/Agencia Española de Cooperación Internacional para el Desarrollo/International
