A new risk score for patients after first recurrence of stage 4 neuroblastoma aged ≥18 months at first diagnosis
- PMID: 31631570
- PMCID: PMC6885891
- DOI: 10.1002/cam4.2562
A new risk score for patients after first recurrence of stage 4 neuroblastoma aged ≥18 months at first diagnosis
Abstract
Background: The prognosis of patients with recurrences from stage 4 neuroblastoma is not uniformly dismal. The evaluation of new therapies therefore needs to consider the individual risks of the treated patients. This study aims to define clinically useful risk criteria.
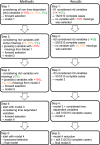
Patients and methods: Inclusion criteria were: first recurrence of neuroblastoma stage 4 aged ≥18 months and enrollment in first line trials between 1997 and 2016. Patients were randomized into a training set (N = 310) and an independent validation set (N = 159). The primary endpoint was secondary event-free survival. The individual treatment elements the patients received during initial and recurrent disease were analyzed as binary and time-dependent variables. A five-step multiple time-dependent Cox regression analysis was performed on the training set to identify prognostic variables adjusted for the individual frontline treatment. The selected variables resulted in a prognostic index (PI) and were used to build a risk score system. The score was validated with the validation set.
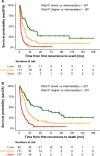
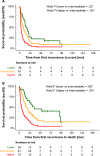
Results: Of the 469 patients, 372 were treated with curative intent and 97 with palliative intent. The PI included the variables number of recurrence organs (hazard ratio [HR] = 2.27), time to recurrence (HR = 2.03), liver metastasis at diagnosis (HR = 1.77), first recurrence at site of the primary tumor (HR = 1.55), and age (HR = 1.29). Three risk groups were built and confirmed in the validation set. The scoring system was likewise useful for the curatively or palliatively treated subgroups.
Conclusion: A new risk score system for patients with first recurrence of stage 4 neuroblastoma aged ≥18 months at diagnosis is proposed.
Keywords: clinical trial; high-risk neuroblastoma; recurrence; relapse; risk score; time-dependent variable.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures

References
-
- Moreno L, Rubie H, Varo A, et al. Outcome of children with relapsed or refractory neuroblastoma: a meta‐analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer. 2017;64(1):25‐31. - PubMed
-
- Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian neuroblastoma registry. Eur J Cancer. 2009;45(16):2835‐2842. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

