Improvement In Self-Reported Physical Functioning With Tiotropium/Olodaterol In Central And Eastern European COPD Patients
- PMID: 31632003
- PMCID: PMC6793952
- DOI: 10.2147/COPD.S204388
Improvement In Self-Reported Physical Functioning With Tiotropium/Olodaterol In Central And Eastern European COPD Patients
Abstract
Background: Reduced physical activity is associated with increased morbidity and mortality in patients with COPD. Studies suggest that treatment with the long-acting muscarinic antagonist tiotropium and the long-acting β2-agonist olodaterol increases exercise capacity. This study assessed the effects of a fixed-dose combination (FDC) of tiotropium/olodaterol (delivered via Respimat®) on physical functioning in patients with stable COPD in a "real-world setting".
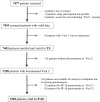
Methods: An international, open-label, single-arm, non-interventional study conducted in nine countries measuring changes in self-reported physical functioning in COPD patients treated with tiotropium/olodaterol 5/5 μg FDC for approximately 6 weeks. The primary endpoint was therapeutic success, defined as a minimum 10-point increase in the 10-question Physical Functioning Questionnaire (PF-10) score. Secondary endpoints included absolute change in PF-10 from Visit 1 to Visit 2, patient general condition (measured by Physician's Global Evaluation score) and patient satisfaction with the treatment and device (assessed by Patient Satisfaction Questionnaire at the end of the study period).
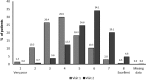
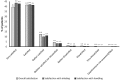
Results: Therapeutic success was observed in 67.8% of 7218 patients (95% CI 66.7, 68.8) in the final analysis set after approximately 6 weeks of treatment with tiotropium/olodaterol. Mean change in PF-10 score between Visit 1 and Visit 2 was 16.6 points (95% CI 16.2, 17.0). Therapeutic success was 64.3% (95% CI 63.0-65.6%) in patients with infrequent (≤1) and 76.1% (95% CI 74.3-77.9%) in patients with frequent (≥2) exacerbations (p<0.0001). Patient general condition improved as indicated by an improvement in Physician's Global Evaluation scores between visits. Most patients were very satisfied or satisfied with tiotropium/olodaterol treatment in general (81%), reported inhalation satisfaction (85%), and satisfactory handling of the device (84%). 1.3% of patients reported an investigator-defined drug-related adverse event.
Conclusion: Treatment with tiotropium/olodaterol led to an improvement in self-reported physical functioning in patients with COPD.
Keywords: COPD; chronic obstructive pulmonary disease; non-interventional study; olodaterol; physical functioning; tiotropium.
© 2019 Valipour et al.
Conflict of interest statement
Dr Arschang Valipour reports personal fees, non-financial support from Boehringer Ingelheim, personal fees from Novartis, Chiesi, and AstraZeneca, during the conduct of the study. Dr Valentina Bayer, Dr Maria Sanzharovskaya, Dr Alexey Medvedchikov are employees of Boehringer Ingelheim. Prof. Dr Zvi Fridlender reports personal fees and institutional support from Boehringer Ingelheim, during the conduct of the study. Prof Dr. Zvi Fridlender also reports personal fees from GSK, Novartis, AstraZeneca, Boehringer Ingelheim, Teva, and Rafa, outside the submitted work. Dr Claudia Toma reports grants, personal fees from Boehringer Ingelheim, during the conduct of the study, personal fees, non-financial support from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Novartis, Roche, Sandoz and Chiesi, personal fees from Amring, Bayer Pharma, Cipla, and Bristol Myers Squibb, and non-financial support from Actelion, Angelini, and Terapia, from outside the submitted work. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Assessment of physical functioning and handling of tiotropium/olodaterol Respimat® in patients with COPD in a real-world clinical setting.Int J Chron Obstruct Pulmon Dis. 2019 Jul 4;14:1441-1453. doi: 10.2147/COPD.S195852. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31308649 Free PMC article.
-
Impact of tiotropium + olodaterol on physical functioning in COPD: results of an open-label observational study.Int J Chron Obstruct Pulmon Dis. 2016 Apr 27;11:891-8. doi: 10.2147/COPD.S103023. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27217742 Free PMC article.
-
Study Design of VESUTO®: Efficacy of Tiotropium/Olodaterol on Lung Hyperinflation, Exercise Capacity, and Physical Activity in Japanese Patients with Chronic Obstructive Pulmonary Disease.Adv Ther. 2017 Jul;34(7):1622-1635. doi: 10.1007/s12325-017-0554-3. Epub 2017 May 23. Adv Ther. 2017. PMID: 28537001 Free PMC article.
-
Tiotropium/Olodaterol: A Review in COPD.Drugs. 2016 Jan;76(1):135-46. doi: 10.1007/s40265-015-0527-2. Drugs. 2016. PMID: 26683033 Review.
-
Tiotropium/Olodaterol: A Review in COPD.Drugs. 2019 Jun;79(9):997-1008. doi: 10.1007/s40265-019-01133-w. Drugs. 2019. PMID: 31119643 Free PMC article. Review.
Cited by
-
COPD in Firefighters: A Specific Event-Related Condition Rather than a Common Occupational Respiratory Disorder.Medicina (Kaunas). 2022 Feb 5;58(2):239. doi: 10.3390/medicina58020239. Medicina (Kaunas). 2022. PMID: 35208563 Free PMC article. Review.
-
An Observational Study Assessing Changes in Health and Functional Status in Patients with Chronic Obstructive Pulmonary Disease (COPD) During Therapy with Spiolto® Respimat® in Everyday Clinical Practice: The Greek ELLACTO Study.Pulm Ther. 2021 Dec;7(2):429-443. doi: 10.1007/s41030-021-00156-7. Epub 2021 May 3. Pulm Ther. 2021. PMID: 33939158 Free PMC article.
-
Targeting exertional breathlessness to improve physical activity: the role of primary care.NPJ Prim Care Respir Med. 2021 Sep 9;31(1):41. doi: 10.1038/s41533-021-00254-8. NPJ Prim Care Respir Med. 2021. PMID: 34504091 Free PMC article. Review.
-
Swiss Experience in Therapy With Dual Bronchodilation in Chronic Obstructive Pulmonary Disease in Relation to Self-Reported Physical Functionality.J Clin Med Res. 2021 Jul;13(7):392-402. doi: 10.14740/jocmr4542. Epub 2021 Jul 28. J Clin Med Res. 2021. PMID: 34394782 Free PMC article.
-
Health and functional status of tiotropium/olodaterol-treated patients with COPD: results from the AERIAL® non-interventional study.ERJ Open Res. 2021 Sep 6;7(3):00004-2021. doi: 10.1183/23120541.00004-2021. eCollection 2021 Jul. ERJ Open Res. 2021. PMID: 34513983 Free PMC article.
References
-
- World Health Organization. Chronic obstructive pulmonary disease (COPD). Available from: http://www.who.int/mediacentre/factsheets/fs315/en/ Accessed February19, 2018.
-
- Corbridge SJ, Nyenhuis SM. Promoting physical activity and exercise in patients with asthma and chronic obstructive pulmonary disease. J Nurse Pract. 2017;13(1):41–46. doi:10.1016/j.nurpra.2016.08.022 - DOI