Nano-Hydroxyapatite Coating Promotes Porous Calcium Phosphate Ceramic-Induced Osteogenesis Via BMP/Smad Signaling Pathway
- PMID: 31632013
- PMCID: PMC6781424
- DOI: 10.2147/IJN.S216182
Nano-Hydroxyapatite Coating Promotes Porous Calcium Phosphate Ceramic-Induced Osteogenesis Via BMP/Smad Signaling Pathway
Abstract
Background: The hierarchical porous structure and surface topography of calcium phosphate (CaP) bioceramics have a crucial impact on their osteoinductivity.
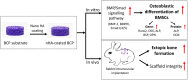
Purpose: To fabricate a biomimetic bone graft with an interconnected porous structure analogous to that of trabecular bone and a bioactive nanostructured surface with excellent osteoinductive potential.
Materials and methods: A biphasic CaP (BCP) substrate with highly porous structure was fabricated by an improved sponge replication method. Surface modification was performed by uniformly depositing a hydroxyapatite (HA) nanoparticle layer to create nHA-coated BCP scaffolds. The effects of these scaffolds on osteogenic differentiation of murine bone marrow-derived stem cells (BMSCs) were investigated in vitro, and their osteoinductivity was further assessed in vivo.
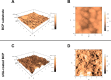
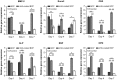
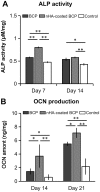
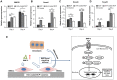
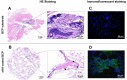
Results: The BCP and nHA-coated BCP scaffolds had similar trabecular bone-like architectures but different surface structures, with mean grain sizes of ~55 nm and ~1 μm, respectively. Compared with the BCP substrate, the nHA-coated BCP scaffolds favored cell adhesion and promoted osteogenic differentiation of BMSCs, as evidenced by upregulated expression of osteogenic genes, enhanced alkaline phosphatase activity, and increased osteocalcin production. This could be attributed to activation of the BMP/Smad signaling pathway, as significantly higher expression levels of BMPRI, Smad1, Smad4, and Smad5 were observed in the nHA-coated BCP group. The nHA-coated BCP scaffold not only maintained scaffold integrity but also induced ectopic bone formation when implanted into rabbit dorsal muscle in vivo for 90 days, whereas the BCP substrate underwent marked biodegradation that led to severe inflammation with no sign of osteogenesis.
Conclusion: The present study demonstrates the potential of this biomimetic bone graft with a trabecular framework and nanotopography for use in orthopedic applications.
Keywords: MSCs; calcium phosphate ceramics; nano-hydroxyapatite; osteoblastic differentiation; osteoinduction; porous scaffolds.
© 2019 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Effect of nano-hydroxyapatite coating on the osteoinductivity of porous biphasic calcium phosphate ceramics.BMC Musculoskelet Disord. 2014 Apr 1;15:114. doi: 10.1186/1471-2474-15-114. BMC Musculoskelet Disord. 2014. PMID: 24690170 Free PMC article.
-
Porous biphasic calcium phosphate ceramics coated with nano-hydroxyapatite and seeded with mesenchymal stem cells for reconstruction of radius segmental defects in rabbits.J Mater Sci Mater Med. 2015 Nov;26(11):257. doi: 10.1007/s10856-015-5590-4. Epub 2015 Oct 8. J Mater Sci Mater Med. 2015. PMID: 26449447
-
Bone morphogenetic protein Smads signaling in mesenchymal stem cells affected by osteoinductive calcium phosphate ceramics.J Biomed Mater Res A. 2015 Mar;103(3):1001-10. doi: 10.1002/jbm.a.35242. Epub 2014 Jun 9. J Biomed Mater Res A. 2015. PMID: 24889783
-
The role of calcium phosphate surface structure in osteogenesis and the mechanisms involved.Acta Biomater. 2020 Apr 1;106:22-33. doi: 10.1016/j.actbio.2019.12.034. Epub 2020 Jan 9. Acta Biomater. 2020. PMID: 31926336 Review.
-
Nano-hydroxyapatite structures for bone regenerative medicine: Cell-material interaction.Bone. 2024 Feb;179:116956. doi: 10.1016/j.bone.2023.116956. Epub 2023 Nov 10. Bone. 2024. PMID: 37951520 Review.
Cited by
-
Nanostructure-Mediated Photothermal Effect for Reinforcing Physical Killing Activity of Nanorod Arrays.Adv Sci (Weinh). 2025 Jan;12(2):e2411997. doi: 10.1002/advs.202411997. Epub 2024 Nov 18. Adv Sci (Weinh). 2025. PMID: 39556665 Free PMC article.
-
Hydroxyapatite Use in Spine Surgery-Molecular and Clinical Aspect.Materials (Basel). 2022 Apr 15;15(8):2906. doi: 10.3390/ma15082906. Materials (Basel). 2022. PMID: 35454598 Free PMC article. Review.
-
Plasma Spray vs. Electrochemical Deposition: Toward a Better Osteogenic Effect of Hydroxyapatite Coatings on 3D-Printed Titanium Scaffolds.Front Bioeng Biotechnol. 2021 Jul 26;9:705774. doi: 10.3389/fbioe.2021.705774. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34381765 Free PMC article.
-
Enhancement of critical-sized bone defect regeneration using UiO-66 nanomaterial in rabbit femurs.BMC Vet Res. 2022 Jul 5;18(1):260. doi: 10.1186/s12917-022-03347-9. BMC Vet Res. 2022. PMID: 35791016 Free PMC article.
-
The Role of Tantalum Nanoparticles in Bone Regeneration Involves the BMP2/Smad4/Runx2 Signaling Pathway.Int J Nanomedicine. 2020 Apr 14;15:2419-2435. doi: 10.2147/IJN.S245174. eCollection 2020. Int J Nanomedicine. 2020. Retraction in: Int J Nanomedicine. 2020 May 13;15:3391. doi: 10.2147/IJN.S261824. PMID: 32368035 Free PMC article. Retracted.
References
-
- Hong Y, Fan H, Li B, Guo B, Liu M, Zhang X. Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Mater Sci Eng R Rep. 2010;70(3–6):225–242. doi:10.1016/j.mser.2010.06.010 - DOI
-
- Jones JR, Hench LL. Regeneration of trabecular bone using porous ceramics. Curr Opin Solid State Mater Sci. 2003;7(4–5):301–307. doi:10.1016/j.cossms.2003.09.012 - DOI
-
- Habibovic P, Sees TM, van Den Doel MA, et al. Osteoinduction by biomaterials – physicochemical and structural influences. J Biomed Mater Res Part A. 2006;77A(4):747–762. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

