CD-340 functionalized doxorubicin-loaded nanoparticle induces apoptosis and reduces tumor volume along with drug-related cardiotoxicity in mice
- PMID: 31632019
- PMCID: PMC6790403
- DOI: 10.2147/IJN.S220740
CD-340 functionalized doxorubicin-loaded nanoparticle induces apoptosis and reduces tumor volume along with drug-related cardiotoxicity in mice
Erratum in
-
Erratum: CD-340 Functionalized Doxorubicin-Loaded Nanoparticle Induces Apoptosis And Reduces Tumor Volume Along With Drug-Related Cardiotoxicity In Mice [Corrigendum].Int J Nanomedicine. 2019 Nov 29;14:9307. doi: 10.2147/IJN.S237385. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31819432 Free PMC article.
-
Erratum: CD-340 Functionalized Doxorubicin-Loaded Nanoparticle Induces Apoptosis and Reduces Tumor Volume Along with Drug-Related Cardiotoxicity in Mice [Corrigendum].Int J Nanomedicine. 2021 Feb 11;16:1001-1003. doi: 10.2147/IJN.S305317. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33603364 Free PMC article.
Abstract
Background and objective: Targeted drug delivery of nanoparticles decorated with site-specific recognition ligands is of considerable interest to minimize cytotoxicity of chemotherapeutics in the normal cells. The study was designed to develop CD-340 antibody-conjugated polylactic-co-glycolic acid (PLGA) nanoparticles loaded with a highly water-soluble potent anticancer drug, doxorubicin (DOX), to specifically deliver entrapped DOX to breast cancer cells.
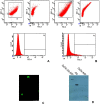
Methods: The study showed how to incorporate water-soluble drug in a hydrophobic PLGA (85:15) based matrix which otherwise shows poor drug loading due to leaching effect. The optimized formulation was covalently conjugated to anti-human epidermal growth factor receptor-2 (HER2) antibody (CD-340). Surface conjugation of the ligand was assessed by flow cytometry, confocal microscopy, and gel electrophoresis. Selectivity and cytotoxicity of the experimental nanoparticles were tested on human breast cancer cells SKBR-3, MCF-7, and MDA-MB-231. Both CD-340-conjugated and unconjugated nanoparticles were undergone in vitro and in vivo characterization.
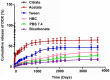
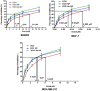
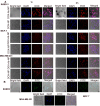
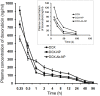
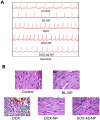
Result: Higher level of incorporation of DOX (8.5% W/W), which otherwise shows poor drug loading due to leaching effect of the highly water-soluble drug, was seen in this method. In HER2-overexpressing tumor xenograft model, radiolabeled antibody-conjugated nanoparticles showed preferentially more of the formulation accumulation in the tumor area when compared to the treatments with the unconjugated one or with the other control groups of mice. The ligand conjugated nanoparticles showed considerable potential in reduction of tumor growth and cardiac toxicity of DOX in mice, a prominent side-effect of the drug.
Conclusion: In conclusion, CD-340-conjugated PLGA nanoparticles containing DOX preferentially delivered encapsulated drug to the breast cancer cells and in breast tumor and reduced the breast tumor cells by apoptosis. Site-specific delivery of the formulation to neoplastic cells did not affect normal cells and showed a drastic reduction of DOX-related cardiotoxicity.
Keywords: breast cancer; ligand; nanoparticles; targeting; tumor.
© 2019 Mondal et al.
Conflict of interest statement
The authors of this article have no conflict of interest to declare with regard to this work.
Figures




References
-
- Alatrash G, Molldrem JJ. Tumor-associated antigens In: Socié G, Blazar BR editors. Immune Biology of Allogeneic Hematopoietic Stem Cell Transplantation.Models in Discovery and Translation. Academic Press; ISBN: 978-0-12-416004-0; 2013. 143–164.
-
- Mukherjee B. Nanosize drug delivery system. Curr Pharm Biotechnol. 2013;14(15):1221. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

