Cullin Ring Ubiquitin Ligases (CRLs) in Cancer: Responses to Ionizing Radiation (IR) Treatment
- PMID: 31632280
- PMCID: PMC6781834
- DOI: 10.3389/fphys.2019.01144
Cullin Ring Ubiquitin Ligases (CRLs) in Cancer: Responses to Ionizing Radiation (IR) Treatment
Abstract
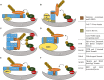
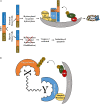
Treatment with ionizing radiation (IR) remains the cornerstone of therapy for multiple cancer types, including disseminated and aggressive diseases in the palliative setting. Radiotherapy efficacy could be improved in combination with drugs that regulate the ubiquitin-proteasome system (UPS), many of which are currently being tested in clinical trials. The UPS operates through the covalent attachment of ATP-activated ubiquitin molecules onto substrates following the transfer of ubiquitin from an E1, to an E2, and then to the substrate via an E3 enzyme. The specificity of ubiquitin ligation is dictated by E3 ligases, which select substrates to be ubiquitylated. Among the E3s, cullin ring ubiquitin ligases (CRLs) represent prototypical multi-subunit E3s, which use the cullin subunit as a central assembling scaffold. CRLs have crucial roles in controlling the cell cycle, hypoxia signaling, reactive oxygen species clearance and DNA repair; pivotal factors regulating the cancer and normal tissue response to IR. Here, we summarize the findings on the involvement of CRLs in the response of cancer cells to IR, and we discuss the therapeutic approaches to target the CRLs which could be exploited in the clinic.
Keywords: DNA-damage; E3-ligases; cullin ring ligases (CRLs); cullins; double-strand breaks (DSBs); ionizing radiation (IR).
Copyright © 2019 Fouad, Wells, Hill and D’Angiolella.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

