Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice
- PMID: 31632356
- PMCID: PMC6779730
- DOI: 10.3389/fmicb.2019.02155
Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice
Abstract
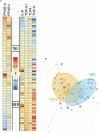
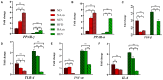
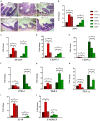
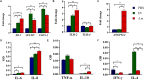
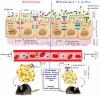
Recent evidence suggests that probiotics can restore the mucosal barrier integrity, ameliorate inflammation, and promote homeostasis required for metabolism in obesity by affecting the gut microbiota composition. In this study, we investigated the effect of Akkermansia muciniphila and its extracellular vesicles (EVs) on obesity-related genes in microarray datasets and evaluated the cell line and C57BL/6 mice by conducting RT-PCR and ELISA assays. A. muciniphila-derived EVs caused a more significant loss in body and fat weight of high-fat diet (HFD)-fed mice, compared with the bacterium itself. Moreover, treatment with A. muciniphila and EVs had significant effects on lipid metabolism and expression of inflammatory markers in adipose tissues. Both treatments improved the intestinal barrier integrity, inflammation, energy balance, and blood parameters (i.e., lipid profile and glucose level). Our findings showed that A. muciniphila-derived EVs contain various biomolecules, which can have a positive impact on obesity by affecting the involved genes. Also, our results showed that A. muciniphila and its EVs had a significant relationship with intestinal homeostasis, which highlights their positive role in obesity treatment. In conclusion, A. muciniphila-derived EVs can be used as new therapeutic strategies to ameliorate HFD-induced obesity by affecting various mechanisms.
Keywords: Akkermansia muciniphila; Angplt4; extracellular vesicles; gut microbiota; obesity; peroxisome proliferator-activated receptors; tight junction; toll-like receptors.
Copyright © 2019 Ashrafian, Shahriary, Behrouzi, Moradi, Keshavarz Azizi Raftar, Lari, Hadifar, Yaghoubfar, Ahmadi Badi, Khatami, Vaziri and Siadat.
Figures

References
-
- Alvarez C.-S., Badia J., Bosch M., Giménez R., Baldomà L. (2016). Outer membrane vesicles and soluble factors released by probiotic Escherichia coli Nissle 1917 and commensal ECOR63 enhance barrier function by regulating expression of tight junction proteins in intestinal epithelial cells. Front. Microbiol. 7:1981. 10.3389/fmicb.2016.01981 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

