Novel multi-drugs incorporating hybrid-structured nanofibers enhance alkylating agent activity in malignant gliomas
- PMID: 31632467
- PMCID: PMC6767748
- DOI: 10.1177/1758835919875555
Novel multi-drugs incorporating hybrid-structured nanofibers enhance alkylating agent activity in malignant gliomas
Abstract
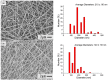
Background: Malignant gliomas (MGs) are highly chemotherapy-resistant. Temozolomide (TMZ) and carmustine (BiCNU) are alkylating agents clinically used for treating MGs. However, their effectiveness is restrained by overexpression of the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) in tumors. O6-benzylguanine (O6-BG) is a nonreversible inhibitor of MGMT, it promotes the cytotoxicity of alkylating chemotherapy. The authors have developed a hybrid-structured nanofibrous membrane (HSNM) that sequentially delivers high concentrations of O6-BG, BiCNU, and TMZ in an attempt to provide an alternative to the current therapeutic options for MGs.
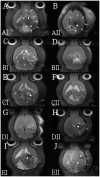
Methods: The HSNMs were implanted onto the cerebral surface of pathogen-free rats following surgical craniectomy, while the in vivo release behaviors of O6-BG, TMZ, and BiCNU from the HSNMs were explored. Subsequently, the HSNMs were surgically implanted onto the brain surface of two types of tumor-bearing rats. The survival rate, tumor volume, malignancy of tumor, and apoptotic cell death were evaluated and compared with other treatment regimens.
Results: The biodegradable HSNMs sequentially and sustainably delivered high concentrations of O6-BG, BiCNU, and TMZ for more than 14 weeks. The tumor-bearing rats treated with HSNMs demonstrated therapeutic advantages in terms of retarded and restricted tumor growth, prolonged survival time, and attenuated malignancy.
Conclusion: The results demonstrated that O6-BG potentiates the effects of interstitially transported BiCNU and TMZ. Therefore, O6-BG may be required for alkylating agents to offer maximum therapeutic benefits for the treatment of MGMT-expressing tumors. In addition, the HSNM-supported chemoprotective gene therapy enhanced chemotherapy tolerance and efficacy. It can, therefore, potentially provide an improved therapeutic alternative for MGs.
Keywords: O6-benzylguanine (O6-BG); O6-methylguanine-DNA methyltransferase (MGMT); chemoresistance; malignant glioma; nanofibrous membrane.
© The Author(s), 2019.
Conflict of interest statement
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Figures









References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008; 359: 492–507. - PubMed
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999; 17: 2572–2578. - PubMed
-
- Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 2008; 62: 564–576; discussion -76. - PubMed
-
- Bobola MS, Tseng SH, Blank A, et al. Role of O6-methylguanine-DNA methyltransferase in resistance of human brain tumor cell lines to the clinically relevant methylating agents temozolomide and streptozotocin. Clin Cancer Res 1996; 2: 735–741. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

