Effects of Tianqijiangtang capsule on survival, self-renewal and differentiation of hippocampal neural stem cells of embryonic rats cultured in high glucose medium
- PMID: 31632529
- PMCID: PMC6789250
Effects of Tianqijiangtang capsule on survival, self-renewal and differentiation of hippocampal neural stem cells of embryonic rats cultured in high glucose medium
Abstract
Objective: This study aims to investigate the effects of Tianqijiangtang capsule on the survival, self-renewal and differentiation of hippocampal neural stem cells (NSCs) of embryonic rats cultured in high glucose medium.
Materials and methods: A cell model of diabetic encephalopathy was established. Cell viability was assessed to screen the optimal concentration of glucose for the cell model of diabetic encephalopathy. Then, the effects of Tianqijiangtang capsule on the proliferation and differentiation of NSCs, and the expression of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) in the culture medium and cells were detected.
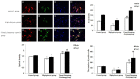
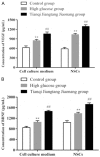
Results: High glucose significantly reduced the ability of survival, proliferation and differentiation of NSCs, which was statistically significant, when compared to the control group (P < 0.05 or 0.01). Tianqijiangtang capsule significantly enhanced the survival, proliferation and differentiation of NSCs cultured in high glucose medium, which was statistically significant, when compared with the high glucose group (P < 0.05 or 0.01). The high glucose culture resulted in a significant decrease in VEGF and BDNF levels in culture medium and cells of NSCs. Tianqijiangtang capsule significantly increased the level of VEGF nuclear BDNF in cells and the culture medium, which was significantly higher, when compared to that in the high glucose group (P < 0.05 or 0.01).
Conclusion: Tianqijiangtang capsule enhances the level of neurotrophic factor synthesized and secreted by hippocampal NSCs cultured with high glucose through the autocrine and paracrine pathway, promotes the NSC survival, replication and differentiation of new neurons and astrocytes, and reduces the degeneration and necrosis of nerve cells.
Keywords: Tianqijiangtang capsule; brain-derived neurotrophic factor; diabetic encephalopathy; neural stem cells; vascular endothelial growth factor.
AJTR Copyright © 2019.
Conflict of interest statement
None.
Figures





References
-
- Khan ZA, Farhangkhoee H, Chakrabarti S. Towards newer molecular targets for chronic diabetic complications. Curr Vasc Pharmacol. 2006;4:45–57. - PubMed
-
- Mijnhout GS, Scheltens P, Diamant M, Biessels GJ, Wessels AM, Simsek S, Snoek FJ, Heine RJ. Diabetic encephalopathy: a concept in need of a definition. Diabetologia. 2006;49:1447–1448. - PubMed
-
- Miles WR, Root HF. Psychologic tests applied in diabetic patients. Arch Intern Med. 1922;30:767–770.
LinkOut - more resources
Full Text Sources
