Loss of HER2 and disease prognosis after neoadjuvant treatment of HER2+ breast cancer
- PMID: 31632579
- PMCID: PMC6789273
Loss of HER2 and disease prognosis after neoadjuvant treatment of HER2+ breast cancer
Abstract
Introduction: HER2 overexpression/amplification occurs in 15-20% breast cancers (BC) and is associated with worse prognosis. The addition of anti-HER2 treatment to neoadjuvant chemotherapy significantly improves the pathological complete response (pCR) rate. Changes in HER2 status after neoadjuvant treatment (NAT) have been reported and may affect prognosis. The aim of this study was to assess the efficacy of NAT in patients with HER2+ BC and its influence on HER2 status and associated prognostic impact.
Methods: Retrospective chart review and pathologic evaluation of all consecutive patients with HER2+ BC (defined as IHC 3+ or IHC 2+ confirmed by SISH) submitted to NAT between 2010-2015 in three Portuguese Hospitals.
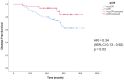
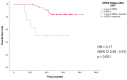
Results: One hundred eight female patients were included; 40 with stage II, 68 with stage III. Hormone receptors were positive in 70. pCR (ypT0/isN0) was achieved in 48 patients (44%). With a median follow-up of 52 months, there were 5 disease free survival (DFS) events among pCR patients and 19 among non-pCR (P = 0.02). Of the 60 patients with residual disease at surgery, 52 remained HER2+ and 8 (13%) lost HER2 overexpression/amplification. 5y-DFS and 5y-OS was 70% and 84%, respectively, for patients whose residual tumors remained HER2+, and 21% and 50% for patients whose residual tumors became HER2 negative (P = 0.02 and < 0.001).
Discussion: We confirmed the negative prognostic impact of NAT-induced HER2 loss on residual tumor leading to worse DFS and OS. Despite the retrospective design and small sample size, these results suggest that it is important to retest HER2 after NAT, to better refine patient outcome.
Keywords: Breast cancer; HER2; chemotherapy; neoadjuvant therapy; trastuzumab.
AJTR Copyright © 2019.
Conflict of interest statement
None.
Figures


References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. - PubMed
-
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. - PubMed
-
- Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, Buchholz T, Meric F, Middleton L, Hortobagyi GN, Gonzalez-Angulo AM. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J. Clin. Oncol. 2006;24:1037–1044. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
