Synthesis and role of melanin for tolerating in vitro rumen digestion in Duddingtonia flagrans, a nematode-trapping fungus
- PMID: 31632832
- PMCID: PMC6781480
- DOI: 10.1080/21501203.2019.1631896
Synthesis and role of melanin for tolerating in vitro rumen digestion in Duddingtonia flagrans, a nematode-trapping fungus
Abstract
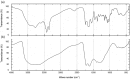
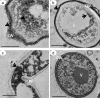
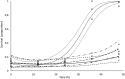
We describe the synthesis and a function of melanin in Duddingtonia flagrans, a nematode-trapping fungus. We tested various culture media treated with L-DOPA, glucose and tricyclazole on fungal growth and melanin distribution using infrared spectroscopy (IS), electron paramagnetic resonance (EPR) and transmission electron microscopy (TEM). In vitro rumen digestion was used to test the environmental stress and then to evaluate the capacity of this fungus to trap nematode larvae. The growth and melanization of the fungus after 21 days of incubation at 30°C were best in Sabouraud dextrose medium. IS indicated the presence of melanin in D. flagrans, with similar bands for commercial melanin used as a control, and assigned the values obtained by EPR (g of 2.0051 ± 0.0001) to the production of melanin by the fungus. TEM indicated that melanin was produced in melanosomes but was not totally inhibited by tricyclazole. Within the limits of experimental error, the predatory activity of fungus treated with tricyclazole was drastically affected after 27 h of in vitro anaerobic stress with rumen inoculum. The deposition of melanin particles on the fungal cell wall contributed to the maintenance of D. flagrans predatory abilities after in vitro anaerobic ruminal stress.
Keywords: Duddingtonia flagrans; L-DOPA; melanin; nematode-trapping fungi; tricyclazole.
© 2019 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Figures





References
-
- Allam NG, El-Zaher EHF.. 2014. Protective role of Aspergillus fumigatus melanin against ultraviolet (UV) irradiation and Bjerkandera adusta melanin as a candidate vaccine against systemic candidiasis. Afr J Biotechnol. 24:6566–6577.
-
- Andrade MC, Hiura E, Fonseca LA, Magri C, Ferraz CCS, Sena FP, Giradi FM. 2016. Utilization of the Fungus Duddingtonia flagrans in Control of Nematode Larvae Development in Equine Stool. Curr Microbiol. 3:829–835.
LinkOut - more resources
Full Text Sources
