Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow
- PMID: 31632905
- PMCID: PMC6783516
- DOI: 10.3389/fonc.2019.00966
Convection-Enhanced Delivery: Connection to and Impact of Interstitial Fluid Flow
Abstract
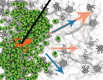
Convection-enhanced delivery (CED) is a method used to increase transport of therapeutics in and around brain tumors. CED works through locally applying a pressure differential to drive fluid flow throughout the tumor, such that convective forces dominate over diffusive transport. This allows therapies to bypass the blood brain barrier that would otherwise be too large or solely rely on passive diffusion. However, this also drives fluid flow out through the tumor bulk into surrounding brain parenchyma, which results in increased interstitial fluid (IF) flow, or fluid flow within extracellular spaces in the tissue. IF flow has been associated with altered transport of molecules, extracellular matrix rearrangement, and triggering of cellular motility through a number of mechanisms. Thus, the results of a simple method to increase drug delivery may have unintended consequences on tissue morphology. Clinically, prediction of dispersal of agents via CED is important to catheter design, placement, and implementation to optimize contact of tumor cells with therapeutic agent. Prediction software can aid in this problem, yet we wonder if there is a better way to predict therapeutic distribution based simply on IF flow pathways as determined from pre-intervention imaging. Overall, CED based therapy has seen limited success and we posit that integration and appreciation of altered IF flow may enhance outcomes. Thus, in this manuscript we both review the current state of the art in CED and IF flow mechanistic understanding and relate these two elements to each other in a clinical context.
Keywords: CED; brain; cancer; drug delivery; fluid flow; glioma; transport.
Copyright © 2019 Stine and Munson.
Figures


References
-
- Brock M, Furuse M, Weber R, Hasuo M, Dietz H. Brain tissue pressure gradients. In: Lundberg N, Pontén U, Brock M. editors. Intracranial Pressure II. Berlin, Heidelberg: Springer Berlin Heidelberg; (1975). p. 215–20. 10.1007/978-3-642-66086-3_45 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

