Why Be One Protein When You Can Affect Many? The Multiple Roles of YB-1 in Lung Cancer and Mesothelioma
- PMID: 31632972
- PMCID: PMC6781797
- DOI: 10.3389/fcell.2019.00221
Why Be One Protein When You Can Affect Many? The Multiple Roles of YB-1 in Lung Cancer and Mesothelioma
Erratum in
-
Corrigendum: Why Be One Protein When You Can Affect Many? The Multiple Roles of YB-1 in Lung Cancer and Mesothelioma.Front Cell Dev Biol. 2019 Nov 22;7:293. doi: 10.3389/fcell.2019.00293. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31807495 Free PMC article.
Abstract
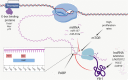
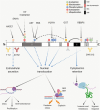
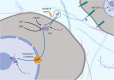
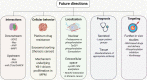
Lung cancers and malignant pleural mesothelioma (MPM) have some of the worst 5-year survival rates of all cancer types, primarily due to a lack of effective treatment options for most patients. Targeted therapies have shown some promise in thoracic cancers, although efficacy is limited only to patients harboring specific mutations or target expression. Although a number of actionable mutations have now been identified, a large population of thoracic cancer patients have no therapeutic options outside of first-line chemotherapy. It is therefore crucial to identify alternative targets that might lead to the development of new ways of treating patients diagnosed with these diseases. The multifunctional oncoprotein Y-box binding protein-1 (YB-1) could serve as one such target. Recent studies also link this protein to many inherent behaviors of thoracic cancer cells such as proliferation, invasion, metastasis and involvement in cancer stem-like cells. Here, we review the regulation of YB-1 at the transcriptional, translational, post-translational and sub-cellular levels in thoracic cancer and discuss its potential use as a biomarker and therapeutic target.
Keywords: Y-box binding protein-1; biomarker; lung cancer; mesothelioma; targeted therapy.
Copyright © 2019 Johnson, Schelch, Mehta, Burgess and Reid.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

