Snap29 mutant mice recapitulate neurological and ophthalmological abnormalities associated with 22q11 and CEDNIK syndrome
- PMID: 31633066
- PMCID: PMC6789041
- DOI: 10.1038/s42003-019-0601-5
Snap29 mutant mice recapitulate neurological and ophthalmological abnormalities associated with 22q11 and CEDNIK syndrome
Abstract
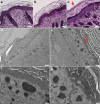
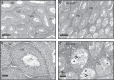
Synaptosomal-associated protein 29 (SNAP29) encodes a member of the SNARE family of proteins implicated in numerous intracellular protein trafficking pathways. SNAP29 maps to the 22q11.2 region and is deleted in 90% of patients with 22q11.2 deletion syndrome (22q11.2DS). Moreover, bi-allelic SNAP29 mutations in patients are responsible for CEDNIK (cerebral dysgenesis, neuropathy, ichthyosis, and keratoderma) syndrome. A mouse model that recapitulates abnormalities found in these syndromes is essential for uncovering the cellular basis of these disorders. In this study, we report that mice with a loss of function mutation of Snap29 on a mixed CD1;FvB genetic background recapitulate skin abnormalities associated with CEDNIK, and also phenocopy neurological and ophthalmological abnormalities found in CEDNIK and a subset of 22q11.2DS patients. Our work also reveals an unanticipated requirement for Snap29 in male fertility and supports contribution of hemizygosity for SNAP29 to the phenotypic spectrum of abnormalities found in 22q11.2DS patients.
Keywords: Disease model; Infertility.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures






References
-
- Fuchs-Telem D, et al. CEDNIK syndrome results from loss-of-function mutations in SNAP29. Br. J. Dermatol. 2011;164:610–616. - PubMed
-
- Sprecher E, et al. A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am. J. Hum. Genet. 2005;77:242–251. doi: 10.1086/432556. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

