Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV
- PMID: 31633805
- PMCID: PMC6802713
- DOI: 10.1002/14651858.CD011420.pub3
Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV
Abstract
Background: The lateral flow urine lipoarabinomannan (LF-LAM) assay Alere Determine™ TB LAM Ag is recommended by the World Health Organization (WHO) to help detect active tuberculosis in HIV-positive people with severe HIV disease. This review update asks the question, "does new evidence justify the use of LF-LAM in a broader group of people?", and is part of the WHO process for updating guidance on the use of LF-LAM.
Objectives: To assess the accuracy of LF-LAM for the diagnosis of active tuberculosis among HIV-positive adults with signs and symptoms of tuberculosis (symptomatic participants) and among HIV-positive adults irrespective of signs and symptoms of tuberculosis (unselected participants not assessed for tuberculosis signs and symptoms).The proposed role for LF-LAM is as an add on to clinical judgement and with other tests to assist in diagnosing tuberculosis.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register; MEDLINE, Embase, Science Citation Index, Web of Science, Latin American Caribbean Health Sciences Literature, Scopus, the WHO International Clinical Trials Registry Platform, the International Standard Randomized Controlled Trial Number Registry, and ProQuest, without language restriction to 11 May 2018.
Selection criteria: Randomized trials, cross-sectional, and observational cohort studies that evaluated LF-LAM for active tuberculosis (pulmonary and extrapulmonary) in HIV-positive adults. We included studies that used the manufacturer's recommended threshold for test positivity, either the updated reference card with four bands (grade 1 of 4) or the corresponding prior reference card grade with five bands (grade 2 of 5). The reference standard was culture or nucleic acid amplification test from any body site (microbiological). We considered a higher quality reference standard to be one in which two or more specimen types were evaluated for tuberculosis diagnosis and a lower quality reference standard to be one in which only one specimen type was evaluated.
Data collection and analysis: Two review authors independently extracted data using a standardized form and REDCap electronic data capture tools. We appraised the quality of studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool and performed meta-analyses to estimate pooled sensitivity and specificity using a bivariate random-effects model and a Bayesian approach. We analyzed studies enrolling strictly symptomatic participants separately from those enrolling unselected participants. We investigated pre-defined sources of heterogeneity including the influence of CD4 count and clinical setting on the accuracy estimates. We assessed the certainty of the evidence using the GRADE approach.
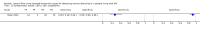
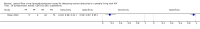
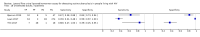
Main results: We included 15 unique studies (nine new studies and six studies from the original review that met the inclusion criteria): eight studies among symptomatic adults and seven studies among unselected adults. All studies were conducted in low- or middle-income countries. Risk of bias was high in the patient selection and reference standard domains, mainly because studies excluded participants unable to produce sputum and used a lower quality reference standard.Participants with tuberculosis symptomsLF-LAM pooled sensitivity (95% credible interval (CrI) ) was 42% (31% to 55%) (moderate-certainty evidence) and pooled specificity was 91% (85% to 95%) (very low-certainty evidence), (8 studies, 3449 participants, 37% with tuberculosis).For a population of 1000 people where 300 have microbiologically-confirmed tuberculosis, the utilization of LF-LAM would result in: 189 to be LF-LAM positive: of these, 63 (33%) would not have tuberculosis (false-positives); and 811 to be LF-LAM negative: of these, 174 (21%) would have tuberculosis (false-negatives).By clinical setting, pooled sensitivity was 52% (40% to 64%) among inpatients versus 29% (17% to 47%) among outpatients; and pooled specificity was 87% (78% to 93%) among inpatients versus 96% (91% to 99%) among outpatients. Stratified by CD4 cell count, pooled sensitivity increased, and specificity decreased with lower CD4 cell count.Unselected participants not assessed for signs and symptoms of tuberculosisLF-LAM pooled sensitivity was 35% (22% to 50%), (moderate-certainty evidence) and pooled specificity was 95% (89% to 96%), (low-certainty evidence), (7 studies, 3365 participants, 13% with tuberculosis).For a population of 1000 people where 100 have microbiologically-confirmed tuberculosis, the utilization of LF-LAM would result in: 80 to be LF-LAM positive: of these, 45 (56%) would not have tuberculosis (false-positives); and 920 to be LF-LAM negative: of these, 65 (7%) would have tuberculosis (false-negatives).By clinical setting, pooled sensitivity was 62% (41% to 83%) among inpatients versus 31% (18% to 47%) among outpatients; pooled specificity was 84% (48% to 96%) among inpatients versus 95% (87% to 99%) among outpatients. Stratified by CD4 cell count, pooled sensitivity increased, and specificity decreased with lower CD4 cell count.
Authors' conclusions: We found that LF-LAM has a sensitivity of 42% to diagnose tuberculosis in HIV-positive individuals with tuberculosis symptoms and 35% in HIV-positive individuals not assessed for tuberculosis symptoms, consistent with findings reported previously. Regardless of how people are enrolled, sensitivity is higher in inpatients and those with lower CD4 cell, but a concomitant lower specificity. As a simple point-of-care test that does not depend upon sputum evaluation, LF-LAM may assist with the diagnosis of tuberculosis, particularly when a sputum specimen cannot be produced.
Conflict of interest statement
Development of the systematic review was, in part, made possible with financial support from the United States Agency for International Development (USAID) administered by the World Health Organization (WHO) Global Tuberculosis Programme, Switzerland. KRS, MS, ND, and SB received funding to carry out the review from USAID.
KRS has also received financial support for the preparation of systematic reviews and educational materials, consultancy fees from FIND (for the preparation of systematic reviews), honoraria, and travel support to attend WHO guideline meetings.
RN is a board member of TB Proof and has participated on an advisory board for Insmed, but these are not thought to represent a conflict of interest.
CMD is employed by FIND, a Swiss non‐profit organization and WHO Collaborating Centre for Diagnosis. FIND provided funding for an initial assessment of data available for the review.
The review authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the review apart from those disclosed.
Figures

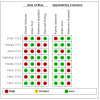
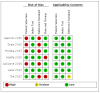



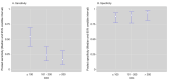




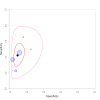




























Update of
References
References to studies included in this review
Bjerrum 2015 {published and unpublished data}
Drain 2015a {published data only}
Drain 2016 {published data only}
Floridia 2017 {published data only}
-
- Floridia M, Ciccacci F, Andreotti M, Hassane A, Sidumo Z, Magid NA, et al. Tuberculosis case finding with combined rapid point‐of‐care assays (Xpert MTB/RIF and Determine TB LAM) in HIV‐positive individuals starting antiretroviral therapy in Mozambique. Clinical Infectious Diseases 2017;65(11):1878‐83. - PubMed
Hanifa 2016 {published data only}
-
- Hanifa Y, Fielding K L, Chihota V N, Adonis L, Charalambous S, Karstaedt A, et al. Diagnostic accuracy of lateral flow urine LAM assay for TB screening of adults with advanced immunosuppression attending routine HIV care in South Africa. PLOS One 2016;11(6):e0156866. [DOI: 10.1371/journal.pone.0156866] - DOI - PMC - PubMed
Huerga 2017 {published data only}
-
- Huerga H, Ferlazzo G, Bevilacqua P, Kirubi B, Ardizzoni E, Wanjala S, et al. Incremental yield of including Determine‐TB LAM assay in diagnostic algorithms for hospitalized and ambulatory HIV‐positive patients in Kenya. PLOS One 2017;12(1):e0170976. [DOI: 10.1371/journal.pone.0170976] - DOI - PMC - PubMed
Juma 2017 {published data only}
-
- Juma FO, Yonga G, Waweru P, Revathi G, Nelson M, Shah R. The diagnostic accuracy of Determine TB LAM antigen in detection of extra pulmonary tuberculosis (EPTB). Journal of Infection 2017;75(6):583‐6. - PubMed
LaCourse 2016 {published and unpublished data}
-
- LaCourse SM, Cranmer LM, Matemo D, Richardson B, Kinuthia J, John‐Stewart G, et al. Active tuberculosis case finding in HIV‐infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. Journal of Acquired Immune Deficiency Syndromes 2016;71(2):219‐27. - PMC - PubMed
Lawn 2017 {published data only}
-
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB‐LAM for routine diagnostic testing for tuberculosis in HIV‐infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Medicine 2017;15(1):67. - PMC - PubMed
Nakiyingi 2014 {published and unpublished data}
Pandie 2016 {published data only}
Peter 2012a {published data only}
Peter 2015 {published data only}
-
- Peter J, Theron G, Chanda D, Clowes P, Rachow A, Lesosky M, et al. Test characteristics and potential impact of the urine LAM lateral flow assay in HIV‐infected outpatients under investigation for TB and able to self‐expectorate sputum for diagnostic testing. BMC Infectious Diseases 2015;15:262. - PMC - PubMed
Peter 2016 {published data only}
-
- Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, et al. Effect on mortality of point‐of‐care, urine‐based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV‐positive hospital inpatients: a pragmatic, parallel‐group, multicountry, open‐label, randomised controlled trial. Lancet 2016;387(10024):1187‐97. - PubMed
References to studies excluded from this review
Agha 2013 {published data only}
-
- Agha MA, El‐Helbawy RH, El‐Helbawy NG, El‐Sheak NM. Utility of quantitative analysis of urine lipoarabinomannan in the diagnosis of tuberculosis. Egyptian Journal of Chest Diseases and Tuberculosis 2013;62(3):401‐7.
Amoudy 1997 {published data only}
-
- Amoudy HA, Al‐Asmer AB, Abul AT, Mustafa AS. Evaluation of complex and defined antigens of Mycobacterium tuberculosis in an IgG‐specific ELISA for the diagnosis of tuberculosis. Medical Principles and Practice 1997;6(2):103‐9.
Andrews 2014 {unpublished data only}
-
- Andrews B, Muchemwa L, Lakhi S, Bwalya M, Mabula C, Chipii G, et al. Validation of a clinical prediction score and performance of urine lipoarabinomannan test for detecting tuberculosis bacteremia in HIV‐positive patients with severe sepsis. American Thoracic Society. 2014:A2559.
Balcha 2014 {published data only}
-
- Balcha TT, Winqvist N, Sturegård E, Skogmar S, Reepalu A, Jemal ZH, et al. Detection of lipoarabinomannan in urine for identification of active tuberculosis among HIV‐positive adults in Ethiopian health centres. Tropical Medicine & International Health 2014;19(6):734‐42. - PubMed
Bisson 2016 {published data only}
-
- Bisson GP, Gupta A, Miyahara S, Sung X, Bao J, Fry CL, et al. Urine LAM testing in advanced HIV‐infected adults in a trial of empiric TB therapy. Topics in Antiviral Medicine. 2016; Vol. 24, issue E‐1:312‐13.
Blanc 2018 {published data only}
-
- Blanc FX, Badje AD, Bonnet M, Gabillard D, Messou E, Muzoora C, et al. Systematic vs test‐guided tuberculosis treatment: Data of the statis randomized trial. Topics in Antiviral Medicine. 2018; Vol. 26, issue Supplement 1:13s‐14s.
Boehme 2005 {published data only}
-
- Boehme C, Molokova E, Minja F, Geis S, Loscher T, Maboko L, et al. Detection of mycobacterial lipoarabinomannan with an antigen‐capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005;99(12):893‐900. - PubMed
Boyles 2012 {published data only}
-
- Boyles TH. The pros and cons of urinary lipoarabinomannan testing. AIDS 2012;26(17):2263‐4; author reply 2264‐5. - PubMed
Broughton 2017 {published data only}
Calligaro 2017 {published data only}
-
- Calligaro Gl, Zijenah LS, Peter JG, Theron G, Buser V, McNerney R, et al. Effect of new tuberculosis diagnostic technologies on community‐based intensified case finding: a multicentre randomised controlled trial. Lancet Infectious Diseases 2017;17(4):441‐50. [DOI: 10.1016/S1473-3099(16)30384-X] - DOI - PubMed
Chan 2000 {published data only}
-
- Chan ED, Reves R, Belisle JT, Brennan PJ, Hahn WE. Diagnosis of tuberculosis by a visually detectable immunoassay for lipoarabinomannan. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1713‐9. - PubMed
Cho 1997 {published data only}
-
- Cho SN, Lee JH, Lee HY, Won HJ, Chong Y, Chang J, et al. Detection of Mycobacterium tuberculosis antigens in sputum for the diagnosis of pulmonary tuberculosis. Journal of the Korean Society for Microbiology 1997;32(3):285‐91.
Conesa‐Botella 2007 {published data only}
-
- Conesa‐Botella A, Loembé MM, Manabe YC, Worodria W, Mazakpwe D, Luzinda K, et al. Urinary lipoarabinomannan as predictor for the tuberculosis immune reconstitution inflammatory syndrome. Journal of Acquired Immune Deficiency Syndromes 2011;58(5):463‐8. - PubMed
Cox 2015 {published data only}
-
- Cox JA, Lukande RL, Kalungi S, Marck E, Vijver K, Kambugu A, et al. Is Urinary Lipoarabinomannan the result of renal tuberculosis? assessment of the renal histology in an autopsy cohort of Ugandan HIV‐infected adults. PLOS One 2015;10(4):e0123323. [DOI: 10.1371/journal.pone.0123323] - DOI - PMC - PubMed
d'Elia 2015 {published data only}
Daley 2009 {published data only}
Deng 2011 {published data only}
-
- Deng S, Yuan T, Xia J, Huang H, Cheng X, Chen M. Clinical utility of a combination of lipoarabinomannan, 38‐kDa, and 16‐kDa antigens as a diagnosis tool for tuberculosis. Diagnostic Microbiology and Infectious Disease 2011;71(1):46‐50. - PubMed
Dheda 2009 {published data only}
Dheda 2010 {published data only}
Drain 2014a {published data only}
Drain 2014b {unpublished data only}
-
- Drain P, Grobler A, Gounder L, Sahid F, Wilson D, Bassett I, et al. Rapid urine lipoarabinomannan testing after two months of tuberculosis treatment independently predicts mortality in a resource‐limited setting. 44th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease. Paris, 2013; Vol. 17 (Suppl 2):S264.
Drain 2015b {published data only}
Drain 2016a {published data only}
Drain 2017 {published data only}
-
- Drain PK, Losina E, Coleman SM, Giddy J, Ross D, Katz JN, et al. Clinic‐based urinary lipoarabinomannan as a biomarker of clinical disease severity and mortality among antiretroviral therapy‐naive human immunodeficiency virus‐infected adults in South Africa. Open Forum Infectious Diseases 2017;4(3):ofx167. [DOI: 10.1093/ofid/ofx167] - DOI - PMC - PubMed
Elsawy 2012 {published data only}
-
- Elsawy A, Redwan EM. Urine lipoarabinomannan as initial markers for active pulmonary tuberculosis. Australian Journal of Basic and Applied Sciences 2012;6(3):751‐5.
Gina 2017 {published data only}
Gounder 2011 {published data only}
-
- Gounder CR, Kufa T, Wada NI, Mngomezulu V, Charalambous S, Hanifa Y, et al. Diagnostic accuracy of a urine lipoarabinomannan enzyme‐linked immunosorbent assay for screening ambulatory HIV‐infected persons for tuberculosis. Journal of Acquired Immune Deficiency Syndromes 2011;58(2):219‐23. - PMC - PubMed
Grant 2016 {published data only}
-
- Grant A, Charalambous S, Tlali M, Johnson S, Dorman S, Hoffmann C, et al. Empirical TB treatment in advanced HIV disease: Results of the TB fast track trial. Topics in Antiviral Medicine. 2016; Vol. 24, issue E‐1:61‐62.
Gupta‐Wright 2016a {published data only}
-
- Gupta‐Wright A, Peters JA, Flach C, Lawn SD. Detection of lipoarabinomannan (LAM) in urine is an independent predictor of mortality risk in patients receiving treatment for HIV‐associated tuberculosis in sub‐Saharan Africa: a systematic review and meta‐analysis. BMC Medicine 2016;14:53. [DOI: 10.1186/s12916-016-0603-9] - DOI - PMC - PubMed
Gupta‐Wright 2016b {published data only}
-
- Gupta‐Wright A, Fielding Kl, Oosterhout JJ, Wilson DK, Corbett El, Flach C, et al. Rapid urine‐based screening for tuberculosis to reduce AIDS‐related mortality in hospitalized patients in Africa (the STAMP trial): study protocol for a randomised controlled trial. BMC Infectious Diseases 2016;16(1):501. [DOI: 10.1186/s12879-016-1837-z] - DOI - PMC - PubMed
Gupta‐Wright 2018b {published data only}
-
- Gupta‐Wright A, Corbett EL, Oosterhout JJ, Wilson DK, Grint, D, Alufandika‐Moyo M, et al. Urine‐based screening for tuberculosis: A randomized trial in HIV‐positive inpatients. Topics in Antiviral Medicine. 2018; Vol. 26, issue Supplement 1:18s‐19s.
Hamasur 2001 {published data only}
-
- Hamasur B, Bruchfeld J, Haile M, Pawlowski A, Bjorvatn B, Källenius G, et al. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. Journal of Microbiological Methods 2001;45(1):41‐52. - PubMed
Hanifa 2015 {published data only}
Iskandar 2017 {published data only}
Kashino 2008 {published data only}
-
- Kashino SS, Pollock N, Napolitano DR, Rodrigues V Jr, Campos‐Neto A. Identification and characterization of Mycobacterium tuberculosis antigens in urine of patients with active pulmonary tuberculosis: an innovative and alternative approach of antigen discovery of useful microbial molecules. Clinical and Experimental Immunology 2008;153(1):56‐62. - PMC - PubMed
Kerkhoff 2014a {published data only}
Kerkhoff 2014b {published data only}
Kerkhoff 2017 {published data only}
-
- Kerkhoff AD, Barr DA, Schutz C, Burton R, Nicol MP, Lawn SD, et al. Disseminated tuberculosis among hospitalised HIV patients in South Africa: a common condition that can be rapidly diagnosed using urine‐based assays. Scientific Reports 2017;7(1):10931. [DOI: 10.1038/s41598-017-09895-7] - DOI - PMC - PubMed
Koralnik 2017 {published data only}
-
- Koralnik IJ, Love S, Mubanga E, Mukondya S, Atadzhanov M, Kosloff B, et al. Optimizing rapid CSF diagnostics of tuberculous meningitis in Zambia. Annals of Neurology. 2017; Vol. 82, issue Supplement 21:S74. [DOI: ]
Koulchin 2007 {published data only}
-
- Koulchin VA, Boehme C, Molokova E, Turecheck C, Hoelscher M, Perkins M, et al. Evaluation of the detection of urinary lipoarabinomannan as a tool for diagnosis of mycobacterial infections. Abstracts of the General Meeting of the American Society for Microbiology 2007;107:174‐5.
Kroidi 2014 {published data only}
-
- Kroidl I, Clowes P, Mwakyelu J, Maboko L, Kiangi A, Rachow A, et al. Reasons for false‐positive lipoarabinomannan ELISA results in a Tanzanian population. Scandinavian Journal of Infectious Diseases 2014;46(2):144‐8. - PubMed
Kroidl 2015 {published data only}
LaCourse 2018a {published data only}
LaCourse 2018b {published data only}
Lawn 2009a {published data only}
-
- Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS 2009;23(14):1875‐80. - PubMed
Lawn 2012a {published data only}
Lawn 2012b {published data only}
-
- Lawn SD, Kerkhoff AD, Vogt M, Wood R. Clinical significance of lipoarabinomannan detection in urine using a low‐cost point‐of‐care diagnostic assay for HIV‐associated tuberculosis. AIDS 2012;26(13):1635‐43. - PubMed
Lawn 2013a {published data only}
Lawn 2014 {unpublished data only}
-
- Lawn SD, Kerkhoff A, Burton R, Schutz C, Wyk G, Vogt M, et al. Massive diagnostic yield of HIV‐associated tuberculosis using rapid urine assays in South Africa. Conference on Retroviruses and Opportunistic Infections (CROI). Boston (MA), 2014.
Leopold 2017 {published data only}
-
- Leopold B, Samuel N, Aimable D, Joannes C. New diagnostic tests for tuberculosis: Performance of LAM and Xpert MTB/RIF in urine of hospitalized patients on intensive phase of TB treatment, and its association with TB dissemination and HIV status. Preliminary results from an observational cohort study in Kigali, Rwanda. Tropical Medicine and International Health. 2017; Vol. 22, issue Supplement 1:35. [DOI: ]
Love 2016 {published data only}
-
- Love S, Mubanga E, Munkondya S, Atadzhanov M, Kosloff B, Ayles H, et al. Optimizing CSF diagnostics of tuberculous meningitis in Zambia. Neurology. 2016; Vol. 86, issue 16 SUPPL. 1.
Manabe 2014 {published data only}
Mathabire 2017 {published data only}
-
- Mathabire SC, Cossa L, Mpunga J, Manhica I, Quiles IA, Molfino L, et al. Feasibility of using Determine‐TB LAM test in HIV‐infected adults in programmatic conditions. Journal of the International Aids Society. 2017; Vol. 20:18.
Mukundan 2012 {published data only}
Musarurwa 2018 {published data only}
-
- Musarurwa C, Zijenah LS, Mhandire DZ, Bandason T, Mhandire K, Chipiti MM, et al. Higher serum 25‐hydroxyvitamin D concentrations are associated with active pulmonary tuberculosis in hospitalised HIV infected patients in a low income tropical setting: a cross sectional study. BMC Pulmonary Medicine 2018;18(1):67. [DOI: 10.1186/s12890-018-0640-6] - DOI - PMC - PubMed
Mutetwa 2009 {published data only}
Nakiyingi 2015 {published data only}
Nakiyingi 2015a {published data only}
-
- Nakiyingi L, Nonyane BA, Ssengooba W, Kirenga BJ, Nakanjako D, Lubega G, et al. Predictors for MTB culture‐positivity among HIV‐infected smear‐negative presumptive tuberculosis patients in Uganda: Application of new tuberculosis diagnostic technology. PLOS One 2015;10(7):e0133756. [DOI: 10.1371/journal.pone.0133756] - DOI - PMC - PubMed
Nicol 2014 {published data only}
Patel 2009 {published data only}
Patel 2010 {published data only}
Peter 2011 {published data only}
-
- Peter JG, Haripesad A, Mottay L, Kraus S, Meldau R, Dheda K. The clinical utility of urine lipoarabinomannan and the novel point‐of‐care lateral flow strip test (Determine TB) for the diagnosis of tuberculosis in hospitalised patients with HIV‐related advanced immunosuppression. American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS 2011;183:A5313.
Peter 2012b {published data only}
Peter 2012c {published data only}
Peter 2013 {published data only}
Reddy 2018 {published data only}
-
- Reddy KP, Gupta‐Wright A, Fielding K, Costantini S, Zheng A, Corbett EL, et al. Cost‐effectiveness of urine TB screening for hospitalized people with HIV in Africa. Topics in Antiviral Medicine. 2018; Vol. 26, issue Supplement 1:518s.
Reid 2015 {unpublished data only}
-
- Reid S, Kancheya N, Kapata N, Kruuner A, Henostroza G, Harris J. Detection of lipoarabinomannan in urine for the diagnosis of tuberculosis in ART clinic enrollees in Lusaka, Zambia. www.cidrz.org/past‐studies/ (accessed 4 September 2015).
Reither 2009 {published data only}
Sabur 2017 {published data only}
Sada 1992 {published data only}
Sahle 2017 {published data only}
-
- Sahle SN, Asress DT, Tullu KD, Weldemariam AG, Tola HH, Awas YA, et al. Performance of point‐of‐care urine test in diagnosing tuberculosis suspects with and without HIV infection in selected peripheral health settings of Addis Ababa, Ethiopia. BMC Research Notes 2017;10(1):74. [DOI: 10.1186/s13104-017-2404-4] - DOI - PMC - PubMed
Savolainen 2013 {published data only}
Schmidt 2011 {published data only}
-
- Schmidt R, Jacak J, Schirwitz C, Stadler V, Michel G, Marmé N, et al. Single‐molecule detection on a protein‐array assay platform for the exposure of a tuberculosis antigen. Journal of Proteome Research 2011;10(3):1316‐22. - PubMed
Shah 2009 {published data only}
Shah 2010 {published data only}
Shah 2013 {published data only}
Shah 2014 {published data only}
Singh 2011 {published data only}
Sun 2013 {published data only}
Suwanpimolkul 2017 {published data only}
-
- Suwanpimolkul G, Kawkitinarong K, Manosuthi W, Sophonphan J, Gatechompol S, Ohata PJ, et al. Utility of urine lipoarabinomannan (LAM) in diagnosing tuberculosis and predicting mortality with and without HIV: prospective TB cohort from the Thailand big city TB research network. International Journal of Infectious Diseases 2017;59:96‐102. [DOI: 10.1016/j.ijid.2017.04.017] - DOI - PubMed
Tessema 2001 {published data only}
-
- Tessema TA, Hamasur B, Bjun G, Svenson S, Bjorvatn B. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scandinavian Journal of Infectious Diseases 2001;33(4):279‐84. - PubMed
Tessema 2002a {published data only}
-
- Tessema TA, Bjune G, Assefa G, Svenson S, Hamasur B, Bjorvatn B. Clinical and radiological features in relation to urinary excretion of lipoarabinomannan in Ethiopian tuberculosis patients. Scandinavian Journal of Infectious Diseases 2002;34(3):167‐71. - PubMed
Tessema 2002b {published data only}
-
- Tessema TA, Bjune G, Hamasur B, Svenson S, Syre H, Bjorvatn B. Circulating antibodies to lipoarabinomannan in relation to sputum microscopy, clinical features and urinary anti‐lipoarabinomannan detection in pulmonary tuberculosis. Scandinavian Journal of Infectious Diseases 2002;34(2):97‐103. - PubMed
Tlali 2014 {unpublished data only}
-
- Tlali M, Fielding K, Charalambous S, Karat A, Hoffmann C, Johnson S, et al. Sensitivity of the TB Determine LAM test compared to sputum culture gold standard TB in ambulant HIV positive participants enrolled in the TB fast track study in South Africa. 4th South African TB Conference. Durban, 2014.
Van Rie 2013 {unpublished data only}
-
- Rie A, Jong E, Mkhwanazi M, Sanne I. Diagnosing TB in those hardest to diagnose: urine lipoarabinomannan for suspects of disseminated and extrapulmonary TB. Conference on Retroviruses and Opportunistic Infections (CROI). Atlanta (GA), 2013:443.
Wood 2012 {published data only}
Zijenah 2016 {published data only}
-
- Zijenah LS, Kadzirange G, Bandason T, Chipiti MM, Gwambiwa B, Makoga F, et al. Comparative performance characteristics of the urine lipoarabinomannan strip test and sputum smear microscopy in hospitalized HIV‐infected patients with suspected tuberculosis in Harare, Zimbabwe. BMC Infectious Diseases 2016; Vol. 16:20. [10.1186/s12879‐016‐1339‐z] - PMC - PubMed
Additional references
Alere 2017
-
- Abbott. Alere DetermineTM TB LAM Ag Product Information. alere.com/en/home/product‐details/determine‐tb‐lam.html (accessed July 18 2017:IN02740004 Rev. 07 2017/08.
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Batz 2011
-
- Batz HG, Cooke GS, Reid SD. Towards lab‐free tuberculosis diagnosis. August 2011. msfaccess.org/sites/default/files/MSF_assets/TB/Docs/TB_Report_TowardsLa.... London: Imperial College, (accessed 16 November 2014).
Boyles 2018
Brennan 2003
-
- Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2003;83(1‐3):91‐7. - PubMed
Briken 2004
-
- Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Molecular Microbiology 2004;53(2):391‐403. - PubMed
Broger 2019
Cain 2010
-
- Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. New England Journal of Medicine 2010;362(8):707‐16. - PubMed
Chu 2006
-
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331–2. - PubMed
Chu 2009
Covidence 2017 [Computer program]
-
- Veritas Health Innovation. Covidence systematic review software. Available at www.covidence.org. Melbourne: Veritas Health Innovation, 2017.
FIND 2018
-
- Foundation for Innovative New Diagnostics (FIND). Call for trial partners now open to advance evaluation of an innovative, sensitive point‐of‐care test that detects tuberculosis in HIV‐positive people. finddx.org/publication/pr‐26sep18/ (accessed 14 June 2019).
Flores 2005
Ford 2016
Getahun 2007
-
- Getahun H, Harrington M, O'Brien R, Nunn P. Diagnosis of smear‐negative pulmonary tuberculosis in people with HIV infection or AIDS in resource‐constrained settings: informing urgent policy changes. Lancet 2007;369(9578):2042‐9. - PubMed
Getahun 2010
-
- Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection—associated tuberculosis: the epidemiology and the response. Clinical Infectious Diseases 2010;50(Suppl 3):S201–7. - PubMed
Getahun 2011
-
- Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, Cain KP, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource‐constrained settings: individual participant data meta‐analysis of observational studies. PLOS Medicine 2011;8(1):e1000391. - PMC - PubMed
GRADEpro 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2015.
Gupta 2012
Gupta 2015
Gupta‐Wright 2018
-
- Gupta‐Wright A, Kerkhoff A D, Meintjes G, Corbett EL. Urinary lipoarabinomannan detection and disseminated nontuberculous mycobacterial disease. Clinical Infectious Diseases 2018;66(1):158. - PubMed
Gupta‐Wright, 2018a
-
- Gupta‐Wright A, Corbett EL, Oosterhout JJ, Wilson D, Grint D, Alufandika‐Moyo M, et al. Rapid urine‐based screening for tuberculosis in HIV‐positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel‐group, double‐blind, randomised controlled trial. Lancet 2018;392:292–301. - PMC - PubMed
Harris 2009
Horne 2019
Kohli 2018
Lawn 2009
Lawn 2012
Lawn 2013
Lawn 2015
Lawn 2016
-
- Lawn SD, Gupta‐Wright A. Detection of lipoarabinomannan (LAM) in urine is indicative of disseminated TB with renal involvement in patients living with HIV and advanced immunodeficiency: evidence and implications. Transactions of The Royal Society of Tropical Medicine and Hygiene 2016;110(3):180‐5. - PMC - PubMed
Lewinsohn 2017
-
- Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official american thoracic society/Infectious diseases society of America/Centers for disease control and prevention clinical practice guidelines: Diagnosis of tuberculosis in adults and children. Clinical Infectious Diseases 2017;64(2):111‐5. - PMC - PubMed
Lunn 2009
-
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique, and future directions. Statistics in Medicine 2009;28:3049‐67. - PubMed
Macaskill 2010
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C (editors), Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from http://srdta.cochrane.org/.
Minion 2011
-
- Minion J, Leung E, Talbot E, Dheda K, Pai M, Menzies D. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta‐analysis. European Respiratory Journal 2011;38(6):1398‐405. - PubMed
Mukora 2018
Nel 2017
-
- Nel JS, Lippincott CK, Berhanu R, Spencer DC, Sanne IM, Ive P. Does disseminated nontuberculous mycobacterial disease cause false‐positive Determine TB‐LAM lateral flow assay results? A retrospective review. Clinincal Infectious Diseases 2017;65(7):1226‐8. - PubMed
Orlando 2018
-
- Orlando S, Triulzi I, Ciccacci F, Palla I, Palombi L, Marazzi MC, et al. Delayed diagnosis and treatment of tuberculosis in HIV+ patients in Mozambique: A cost‐effectiveness analysis of screening protocols based on four symptom screening, smear microscopy, urine LAM test and Xpert MTB/RIF. PLOS One 2018;13(7):e0200523. - PMC - PubMed
Pai 2012
-
- Pai NP, Pai M. Point‐of‐care diagnostics for HIV and tuberculosis: landscape, pipeline, and unmet needs. Discovery Medicine 2012;13(68):35‐45. - PubMed
Pai 2016
-
- Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nature Reviews Disease Primers 2016;2:16076. - PubMed
Perkins 2007
-
- Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. Journal of Infectious Diseases 2007;196(Suppl 1):S15‐27. - PubMed
Peter 2010
Qvist 2014
R Statistical Computing 2018 [Computer program]
-
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018.
Reddy 2019
Reitsma 2005
-
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2008
Schünemann 2016
-
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso‐Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016 August;76:89‐98. - PubMed
SDU Open
-
- Odense Patient data Explorative Network. OPEN. sdu.dk/en/Om_SDU/Institutter_centre/Klinisk_institut/Forskning/Forskning... (accessed 10 June 2018).
Shivakoti 2017
Spiegelhalter 2004
-
- Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health Care Evaluation. New York: Wiley, 2004.
StataCorp 2017 [Computer program]
-
- StataCorp. Stata Statistical Software. Version 13. Texas: StataCorp LP, 2017.
Steingart 2006
-
- Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, Cunningham J, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infectious Diseases 2006;6(9):570‐81. - PubMed
TB CARE I 2014
-
- TB CARE I. International Standards for Tuberculosis Care, Edition 3. TB Care 1, The Hague. who.int/tb/publications/ISTC_3rdEd.pdf?ua=1 (accessed 16 Nov 2014):1‐87.
Unitaid 2017
-
- D Boyle. Tuberculosis Diagnostics Technology and Market Landscape. 5th Edition. Vernier: World Health Organization Unitaid Secretariat, 2017.
Weyer 2011
-
- Weyer K, Carai S, Nunn P. Viewpoint TB diagnostics: what does the world really need?. Journal of Infectious Diseases 2011;204(Suppl 4):S1196‐202. - PubMed
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529–36. - PubMed
WHO ART Guidelines 2016
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd Edition. Geneva: World Health Organization, 2016. - PubMed
WHO Global Tuberculosis Report 2018
-
- World Health Organization. Global Tuberculosis Report 2018. Licence: CC BY‐NC‐SA 3.0 IGO. Geneva: World Health Organization, 2018.
WHO ICF 2011
-
- World Health Organization Stop TB Dept Dept of HIV/AIDS. Guidelines for intensified tuberculosis case‐finding and isoniazid preventive therapy for people living with HIV in resource‐constrained settings. Geneva: World Health Organization, 2011.
WHO Lipoarabinomannan Policy Guidance 2015
-
- World Health Organization. The use of lateral flow urine lipoarabinomannan assay (LF‐LAM) for the diagnosis and screening of active tuberculosis in people living with HIV. Policy guidance. WHO/HTM/TB/2015.25. Geneva: World Health Organization, 2015.
WHO Managing Advanced HIV Disease 2017
-
- World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: World Health Organization, 2017. - PubMed
WHO TB‐LAMP 2016
-
- World Health Organization. The use of Loop‐mediated Isothermal Amplification (TB‐LAMP) for the diagnosis of pulmonary tuberculosis: Policy guidance. Geneva: World Health Organization, 2016. - PubMed
WHO TTP 2014
-
- World Health Organization. High‐priority target product profiles for new tuberculosis diagnostics, Report of a consensus meeting. Geneva: World Health Organization, 2014:98.
WHO Xpert Ultra 2017
-
- World Health Organization. WHO meeting report of a technical expert consultation: non‐inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. Geneva: World Health Organization, 2017.
WHO Xpert® Policy Update 2013
-
- World Health Organization. Automated real‐time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. WHO/HTM/TB/2013.16. Geneva: World Health Organization, 2013. - PubMed
World Bank 2018
-
- World Bank. World Bank Country Classifications by Income. Washington (District of Columbia): World Bank, 2018.
Yuan 2014
-
- Yuan LY, Li Y, Wang M, Ke ZQ, Xu WZ. Rapid and effective diagnosis of pulmonary tuberculosis with novel and sensitive loop‐mediated isothermal amplification (LAMP) assay in clinical samples: a meta‐analysis. Journal of Infection and Chemotherapy 2014;20(2):86‐92. - PubMed
References to other published versions of this review
Shah 2014
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

